Risk of skin cancer development cut almost 75 percent in clinical trial participants who received immunotherapy treatment
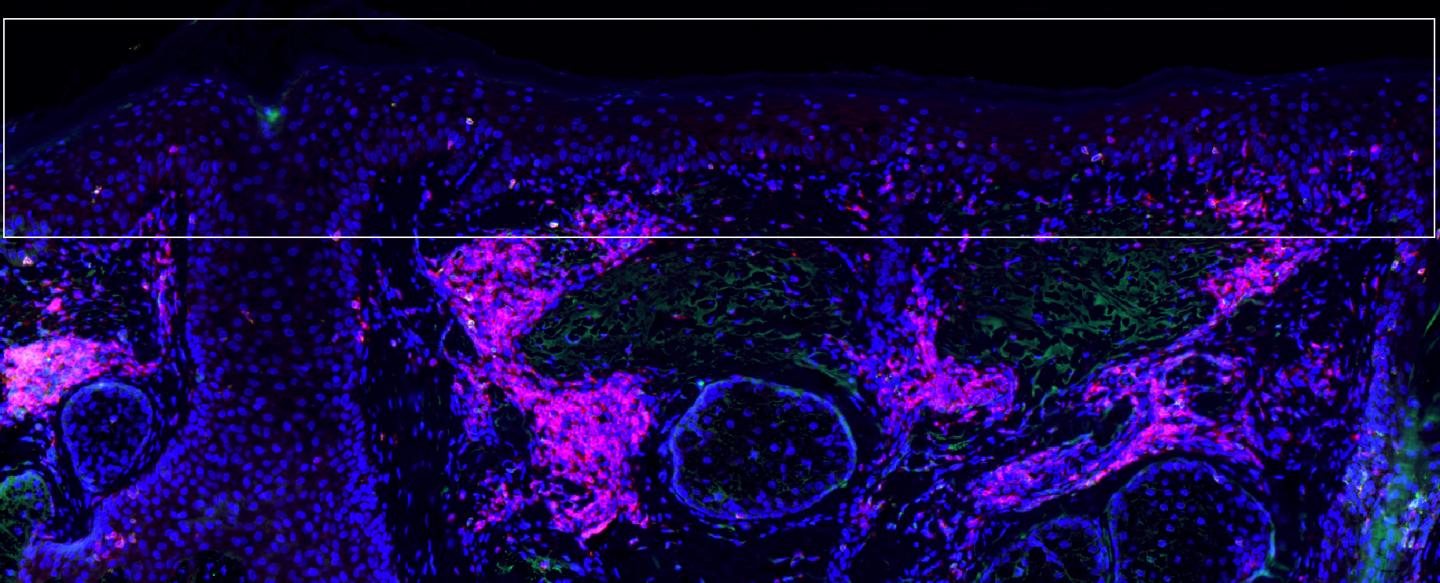
Credit: Kenneth Ngo, Massachusetts General Hospital Center for Cancer Immunology/Cutaneous Biology Research Center
A treatment previously shown to clear the precancerous skin lesions called actinic keratosis now appears to reduce the chance that the treated skin will develop squamous cell carcinomas (SCCs), the second most common form of skin cancer. In their paper being published online in JCI Insight, a team led by a Massachusetts General Hospital (MGH) investigator reports that treatment with the combination of two FDA-approved drugs – a topical chemotherapy and an immune-system-activating compound – reduced the risk of SCC development on the face and scalp by almost 75 percent.
“This finding provides the first clinical proof-of-concept that an immunotherapy directed against premalignant tumors can prevent cancer,” says Shawn Demehri, MD, PhD, of the MGH Center for Cancer Immunology and the Cutaneous Biology Research Center (http://www.
A common lesion that develops in sun-damaged skin, actinic keratosis is the third most common reason for U.S. patients to consult a dermatologist. While the chance of an individual lesion progressing to cancer is only 1 percent, the majority of SCCs develop from existing actinic keratosis lesions. In a study) published in the January 2017 issue of the Journal of Clinical Investigation, Demehri and his team reported that twice-daily treatment for four days with a combination of calcipotriol, an FDA-approved treatment to treat psoriasis, and 5-fluorouracil (5-FU) cream, a standard treatment for actinic keratosis, significantly reduced the number and size of actinic keratosis lesions.
The current study follows up on the participants of the 2017 trial to determine whether or not the combined treatment reduced the risk of their developing SCCs. Of the 130 participants in original trial – 64 who received calcipotriol plus 5-FU and 66 who received a control treatment of 5-FU in petroleum jelly – three-year outcome results were available for 84 individuals, 39 who received the combination treatment and 45, the control treatment. Results for treatment on the face and scalp were available for 72 participants (32 combination treatment and 40 controls).
Overall, a greater proportion of participants who received the combination treatment for lesions on their face and scalp remained free of SCC development for more than three years after treatment, with only 7 percent developing SCC compared with 28 percent in the control group. Samples of treated face and scalp tissues of 22 participants showed higher levels of tissue-resident memory T cells – both CD4+ and CD8+ T cells – in normal skin treated with the combination treatment, compared with the control group.
While treatment did not reduce SCC development on the arms, the investigators believe that may result from greater immune system activation on the face and scalp along with better penetration of topical treatments on those areas. They hypothesize that longer treatment with calcipotriol plus 5-FU may be required to reduce SCC risk on the arms and other parts of the body.
“Our previous findings that calcipotriol plus 5-FU is highly effective for actinic keratosis clearance has led to its being increasingly used in clinics around the world, although further clinical studies are required for formal FDA approval,” says Demehri, an assistant professor of Dermatology at Harvard Medical School. “The treatment of SCC can be costly and has significant side effects, and it can be deadly, particularly for patients with suppressed immune systems.
“The primary reason for treating actinic keratosis is to prevent SCC development, and our findings suggest this immunotherapy may be an effective way of achieving that goal,” he adds. “Finding that targeting precancerous lesions with a robust T-cell-directed immunotherapy can yield effective cancer prevention may be applicable to other organs than the skin, something we hope to investigate for malignancies such as breast cancer, for which immunoprevention is an urgent, unmet need.”
###
The lead author of the JCI Insight report is Abby Rosenberg of the Division of Dermatology at Washington University School of Medicine in St. Louis. Additional co-authors are Mary Tabacchi, Michael Wallendorf, MD, Ilana Rosman, MD, and Lynn Cornelius, MD, Washington University School of Medicine; and Kenneth Ngo, MGH Center for Cancer Immunology and the Cutaneous Biology Research Center. The study was supported by National Institutes of Health grants K08 AR068619 and DP5 OD0213530 and grants from the Burroughs Wellcome Fund, the Sidney Kimmel Foundation and the Cancer Research Institute. Cornelius and Demehri are co-inventors on a filed patent for calcipotriol plus 5-FU to treat precancerous skin lesions.
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The MGH Research Institute conducts the largest hospital-based research program in the nation, with an annual research budget of more than $925 million and major research centers in HIV/AIDS, cardiovascular research, cancer, computational and integrative biology, cutaneous biology, genomic medicine, medical imaging, neurodegenerative disorders, regenerative medicine, reproductive biology, systems biology, photomedicine and transplantation biology. The MGH topped the 2015 Nature Index list of health care organizations publishing in leading scientific journals and earned the prestigious 2015 Foster G. McGaw Prize for Excellence in Community Service. In August 2018 the MGH was once again named to the Honor Roll in the U.S. News & World Report list of “America’s Best Hospitals.”
Media Contact
Katie Marquedant
[email protected]
Related Journal Article
http://dx.




