Study findings lead to accelerated FDA drug approval
Credit: Johns Hopkins Kimmel Cancer Center
The first study of the immunotherapy drug pembrolizumab as the initial treatment for patients with a rare but aggressive form of skin cancer known as Merkel cell carcinoma reports better responses and longer survival than expected with conventional chemotherapy.
The study, co-led by Suzanne Topalian, M.D., associate director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy at the Johns Hopkins Kimmel Cancer Center, is the longest observation to date of Merkel cell carcinoma patients treated with any anti-PD-1 immunotherapy drug used in the first line. The findings, published in the Journal of Clinical Oncology, supported the recent (Dec. 19, 2018) U.S. Food and Drug Administration accelerated approval of pembrolizumab, marketed as Keytruda, as a first-line treatment for adult and pediatric patients with advanced Merkel cell carcinoma.
For this study, investigators from the Bloomberg~Kimmel Institute collaborated with researchers from the Fred Hutchinson Cancer Research Center in Seattle, along with 11 other U.S. medical centers. The Bloomberg~Kimmel Institute team includes Topalian, William Sharfman, M.D., Evan Lipson, M.D., Abha Soni, D.O., M.P.H., and Janis Taube, M.D., M.Sc.
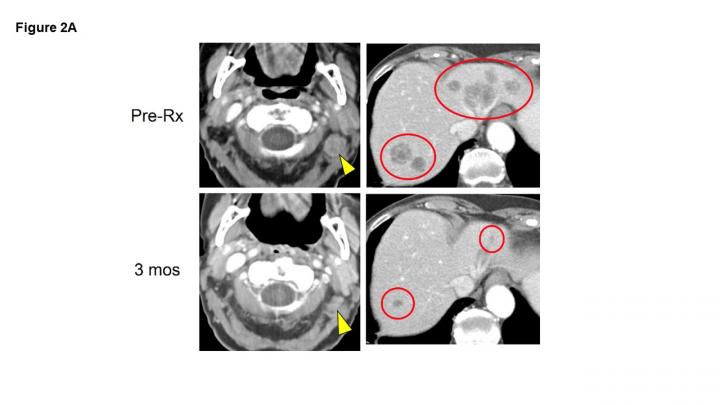
In the 50-patient study of pembrolizumab as the initial treatment for patients with recurrent, locally advanced or metastatic Merkel cell carcinoma, more than half of the patients (28 patients, 56 percent) had long-lasting responses to the treatment, 12 of whom (24 percent) experienced a complete disappearance of their tumors. Nearly 70 percent of patients in this study were alive two years after starting treatment.
“This is the earliest trial of immunotherapy as a front-line therapy for Merkel cell carcinoma, and it was shown to be more effective than what would be expected from traditional therapies, like chemotherapy,” says Topalian, who is a Bloomberg~Kimmel professor of cancer immunotherapy at the Kimmel Cancer Center. “Immunotherapy provides an effective treatment for patients with Merkel cell carcinoma who before had few options. Immunotherapy is unique in cancer treatment, because it does not directly target cancer cells but rather removes constraints on the immune system’s natural ability to find and destroy cancer cells.”
The 50 patients in this study were treated at 13 centers across the United States in a clinical trial conducted by the Cancer Immunotherapy Trials Network, which is sponsored by the National Cancer Institute (NCI). The Kimmel Cancer Center, where much of the medical science contributing to the development of pembrolizumab unfolded, was a lead institution. Preliminary findings regarding the first 26 patients enrolled in the study were published in the New England Journal of Medicine in 2016. The study was subsequently amended to add 24 more patients.
The National Institutes of Health (NIH) classifies Merkel cell carcinoma as an “orphan disease,” as it is diagnosed in fewer than 2,000 people annually in the United States. It typically occurs in older people and those who have suppressed immune systems. About 80 percent of Merkel cell carcinomas are caused by a virus called the Merkel cell polyomavirus. The remaining cases are attributed to ultraviolet light exposure and other, unknown factors.
In the study, treatment with pembrolizumab worked well against both virus-positive and virus-negative Merkel cell carcinomas, resulting in high response rates and durable progression-free survival in both subtypes. The findings also showed that tumors expressing a PD-1-related protein called PD-L1 tended to respond longer to treatment, although patients whose tumors did not express PD-L1 also responded.
“These findings could be a precursor to developing more effective treatments for other virus-related cancers, which account for about 20 percent of cancers worldwide,” says Sharfman, the Mary Jo Rogers Professor of Cancer Immunology and Melanoma Research.
The non-virus-related subtype is characterized by high numbers of genetic mutations in cancer cells, which has also been shown by the Bloomberg~Kimmel Institute group to be a biomarker of response in various cancers to checkpoint inhibitors such as pembrolizumab.
Pembrolizumab works against Merkel cell carcinoma by blocking PD-1, a molecule on the surface of immune cells that regulates immune responses, turning them on and off. Cancer cells often manipulate PD-1 to send a “stop” signal to the immune system. Blocking that signal with a checkpoint inhibitor, such as pembrolizumab, initiates a “go” signal, sending immune cells to attack cancer cells. A protein on the surface of cancer cells, called PD-L1, is one mechanism cancer cells use to manipulate PD-1 and disrupt the immune response.
“Under the microscope, PD-L1 looks like an armor around the cancer cell,” says Taube, an associate professor of oncology, dermatology and pathology. “Pembrolizumab interrupts PD-1 signaling by blocking the communication between PD-1 and PD-L1, removing the stop signal and re-engaging the immune system to go after cancer cells.”
Patients in the just-published study received the immune checkpoint blocking drug pembrolizumab intravenously every three weeks for up to two years. During this time, the status of their cancer was monitored periodically with imaging scans. Overall, most patients tolerated the treatment well. However, 28 percent of patients experienced serious side effects, including one treatment-associated death.
###
Paul Nghiem, M.D., Ph.D., affiliate investigator in the clinical research division at the Fred Hutchinson Cancer Research Center in Seattle, and professor of medicine in the division of dermatology at the University of Washington School of Medicine, is the principal investigator and first author of the study.
In addition to Topalian, Sharfman, Taube, Lipson, Soni and Nghiem, study investigators included Shailender Bhatia, Ragini Kudchadkar, Andrew Brohl, Phillip Friedlander, Adil Daud, Harriet Kluger, Sunil Reddy, Brian Boulmay, Adam Riker, Melissa Burgess, Brent Hanks, Thomas Olencki, Kim Margolin, Lisa Lundgren, Nirasha Ramchurren, Candice Church, Song Park, Michi Shinohara, Bob Salim, Steven Bird, Nageatte Ibrahim, Steven Fling, Blanca Homet-Moreno, Elad Sharon and Martin Cheever.
The research was supported by the National Cancer Institute (1U01CA154967, K24CA139052, R01CA162522, R01CA142779, T32CA193145), the NIH/NCI Cancer Center Support Grant in Seattle (P30CA015704), Merkel cell carcinoma patient gift fund at the University of Washington, Kelsey Dickson Merkel cell carcinoma challenge grant from the Prostate Cancer Foundation, and Merck.
COI: Lipson reports a consulting or advisory role: Bristol-Myers Squibb, Novartis, EMD Serono, Array BioPharma, Regeneron/Sanofi Genzyme, MacroGenics, Merck, Millennium; research funding: Bristol-Myers Squibb (Inst), Merck (Inst), Sysmex (Inst); patents, royalties, other intellectual property: Method of preventing organ transplantation rejections using agonists to the PD-1 checkpoint pathway (Inst). Sharfman reports honoraria: Bristol-Myers Squibb; consulting or advisory role: Merck, Bristol-Myers Squibb, Novartis, Regeneron; research funding: Bristol-Myers Squibb (Inst), Merck, Novartis. Taube reports consulting or advisory role: Bristol-Myers Squibb, AstraZeneca, Merck, Amgen; research funding: Bristol-Myers Squibb; travel, accommodations, expenses: Bristol-Myers Squibb, AstraZeneca, Merck, Amgen. Topalian reports stock and other ownership interests: Aduro Biotech (I), Compugen (I), Potenza Therapeutics (I), Jounce Therapeutics (I), Five Prime Therapeutics, Tizona Therapeutics (I), DNAtrix (I), FLX Bio (I), WindMIL (I), Dragonfly Therapeutics, Ervaxx (I); consulting or advisory role: Five Prime Therapeutics, Amgen (I), MedImmune (I), Merck, AbbVie, Compugen (I), DNAtrix (I), FLX Bio (I), Tizona Therapeutics (I), WindMIL (I), Dragonfly Therapeutics, Bayer (I), Dynavax (I), Ervaxx (I), Immunomic Therapeutics (I), Janssen Oncology (I); research funding: Bristol-Myers Squibb, Compugen (I), Potenza Therapeutics (I); patents, royalties, other intellectual property: Aduro Biotech (I), Bristol-Myers Squibb (I), Immunomic Therapeutics (I); travel, accommodations, expenses: Bristol-Myers Squibb, Five Prime Therapeutics. (I) = immediate family member, (Inst) = my institution.
Potential conflicts of interest reported by other authors can be found at ascopubs.org.
Media Contact
Larry Frum
[email protected]
443-287-2539
Original Source
https:/
Related Journal Article
http://dx.




