Credit: Yen Strandqvist/Chalmers
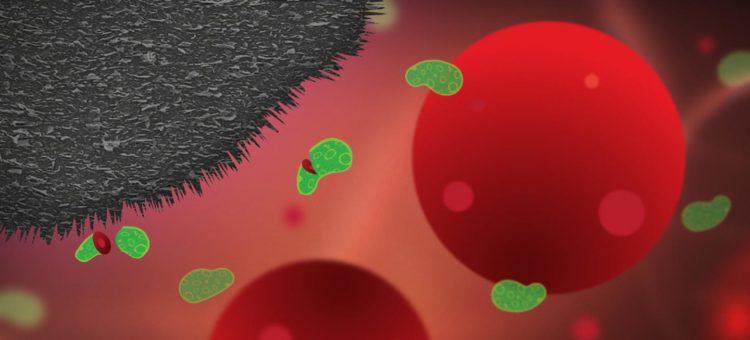
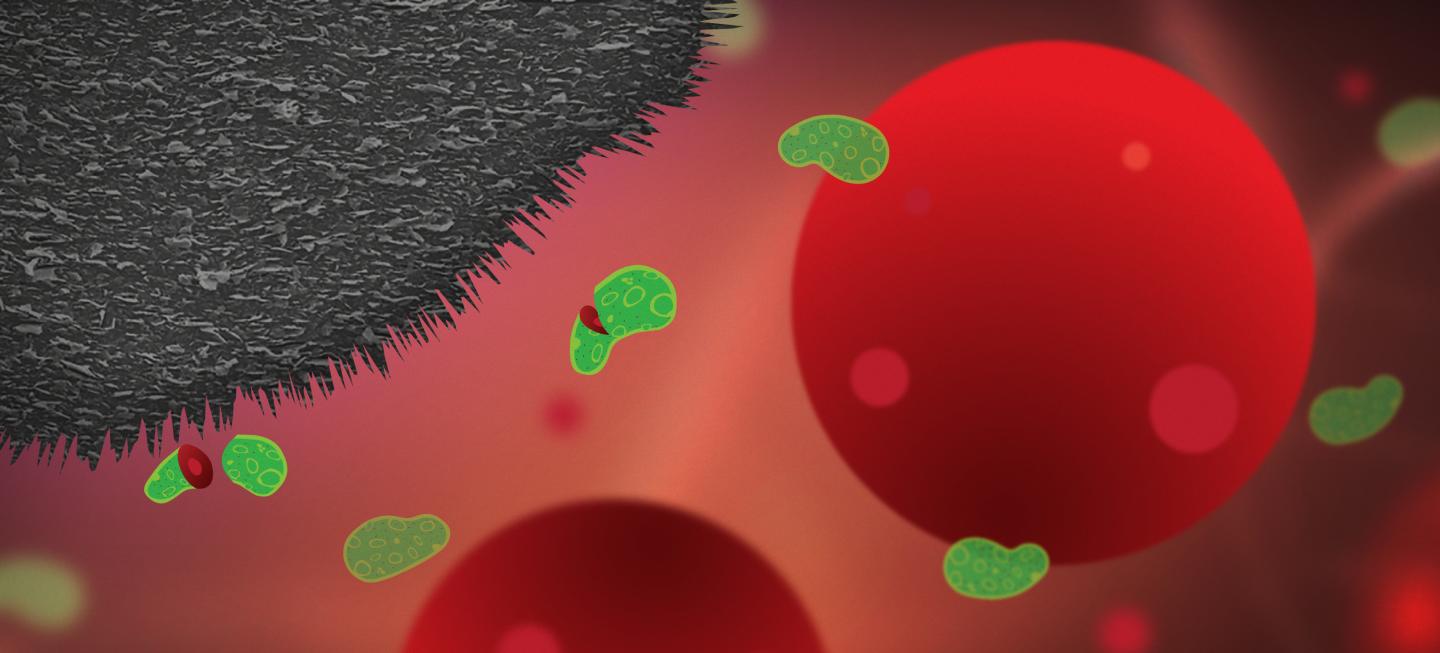
Graphite nanoplatelets integrated into plastic medical surfaces can prevent infections, killing 99.99 per cent of bacteria which try to attach – a cheap and viable potential solution to a problem which affects millions, costs huge amounts of time and money, and accelerates antibiotic resistance. This is according to research from Chalmers University of Technology, Sweden, in the journal Small.
Every year, over four million people in Europe are affected by infections contracted during health-care procedures, according to the European Centre for Disease Prevention and Control (ECDC). Many of these are bacterial infections which develop around medical devices and implants within the body, such as catheters, hip and knee prostheses or dental implants. In worst cases implants need to be removed.
Bacterial infections like this can cause great suffering for patients, and cost healthcare services huge amounts of time and money. Additionally, large amounts of antibiotics are currently used to treat and prevent such infections, costing more money, and accelerating the development of antibiotic resistance.
“The purpose of our research is to develop antibacterial surfaces which can reduce the number of infections and subsequent need for antibiotics, and to which bacteria cannot develop resistance. We have now shown that tailored surfaces formed of a mixture of polyethylene and graphite nanoplatelets can kill 99.99 per cent of bacteria which try to attach to the surface,” says Santosh Pandit, postdoctoral researcher in the research group of Professor Ivan Mijakovic at the Division of Systems Biology, Department of Biology and Biotechnology, Chalmers University of Technology.
Infections on implants are caused by bacteria that travel around in the body in fluids such as blood, in search of a surface to attach to. When they land on a suitable surface, they start to multiply and form a biofilm – a bacterial coating.
Previous studies from the Chalmers researchers showed how vertical flakes of graphene, placed on the surface of an implant, could form a protective coating, making it impossible for bacteria to attach – like spikes on buildings designed to prevent birds from nesting. The graphene flakes damage the cell membrane, killing the bacteria. But producing these graphene flakes is expensive, and currently not feasible for large-scale production.
“But now, we have achieved the same outstanding antibacterial effects, but using relatively inexpensive graphite nanoplatelets, mixed with a very versatile polymer. The polymer, or plastic, is not inherently compatible with the graphite nanoplatelets, but with standard plastic manufacturing techniques, we succeeded in tailoring the microstructure of the material, with rather high filler loadings, to achieve the desired effect. And now it has great potential for a number of biomedical applications,” says Roland Kádár, Associate Professor at the Department of Industrial and Materials Science at Chalmers.
The nanoplatelets on the surface of the implants prevent bacterial infection but, crucially, without damaging healthy human cells. Human cells are around 25 times larger than bacteria, so while the graphite nanoplatelets slice apart and kill bacteria, they barely scratch a human cell.
“In addition to reducing patients’ suffering and the need for antibiotics, implants like these could lead to less requirement for subsequent work, since they could remain in the body for much longer than those used today,” says Santosh Pandit. “Our research could also contribute to reducing the enormous costs that such infections cause health care services worldwide.”
In the study, the researchers experimented with different concentrations of graphite nanoplatelets and the plastic material. A composition of around 15-20 per cent graphite nanoplatelets had the greatest antibacterial effect – providing that the morphology is highly structured.
“As in the previous study, the decisive factor is orienting and distributing the graphite nanoplatelets correctly. They have to be very precisely ordered to achieve maximum effect,” says Roland Kádár.
###
The study was a collaboration between the Division of Systems and Synthetic Biology at the Department of Biology and Biological Engineering, and the Division of Engineering Materials at the Department of Industrial and Materials Science at Chalmers, and the medical company Wellspect Healthcare, who manufacture catheters, among other things. The antibacterial surfaces were developed by Karolina Gaska when she was a postdoctoral researcher in the group of Associate Professor Roland Kádár.
The researchers’ future efforts will now be focused on unleashing the full potential of the antibacterial surfaces for specific biomedical applications.
Read the scientific article, Pre-controlled Alignment of Graphite Nanoplatelets in Polymeric Composites Prevents Bacterial Attachment in the journal Small.
Read the previous press release from April 2018, Spikes of graphene can kill bacteria on implants
For more information contact:
Santosh Pandit
Postdoctoral researcher at the Department of Biology and Biotechnology, Chalmers:
+46 729 48 40 11
[email protected]
Roland Kádár
Associate Professor at the Department of Industry and Materials Science, Chalmers:
+46 031 772 12 56
[email protected]
Ivan Mijakovic
Professor at the Department of Biology and Biotechnology, Chalmers:
[email protected]
Media Contact
Joshua Worth
[email protected]
46-317-726-379
Original Source
http://www.
Related Journal Article
http://dx.