In an increasingly cancer-conscious world, new research findings are pointing towards a promising therapeutic strategy to tackle one of the most aggressive forms of cancer: pancreatic cancer. A collaborative study led by Kang et al. has provided groundbreaking insights into the biochemical pathways that underlie the resistance of pancreatic cancer cells to gemcitabine, a commonly used chemotherapeutic agent. Central to this study is the discovery of the SLC6A14 protein’s role in mediating glutamine uptake, which in turn promotes activation of the SYTL4–CXCL8 axis, shedding light on the mechanisms of immune evasion and drug resistance in cancer therapy.
Gemcitabine has long been a cornerstone treatment for pancreatic cancer, but its efficacy is often significantly reduced due to the rapid development of chemoresistance. This study delves deep into understanding the molecular interactions that confer this resistance, emphasizing the critical function of the SLC6A14 transporter protein. SLC6A14 is known to facilitate the uptake of various amino acids, and its overexpression has been correlated with several malignancies. The authors of the study propose a direct connection between glutamine metabolism fueled by SLC6A14 and the aggressive nature of pancreatic cancer cells.
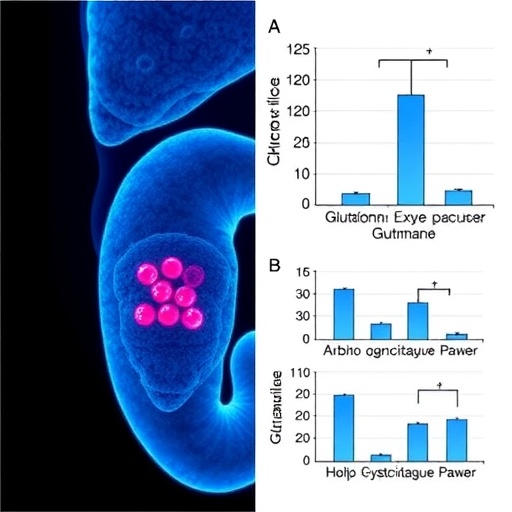
The researchers embarked on an investigation into how the alteration of amino acid transport influences tumor growth and chemotherapy resistance. Through a series of in vitro experiments, they demonstrated that the inhibition of SLC6A14 led to a significant reduction in pancreatic cancer cell proliferation and increased susceptibility to gemcitabine. This finding lays the groundwork for evaluating SLC6A14 as a potential therapeutic target, providing the cancer community with a new avenue for intervention.
In parallel with these findings, the study highlights the role of the SYTL4–CXCL8 axis, a pathway implicated in immune response and inflammation. The authors unveiled that glutamine-mediated signaling activates this axis, enabling cancer cells to evade immune detection. Understanding this immunological aspect is crucial in the fight against pancreatic cancer, which has a high propensity for immune evasion. The activation of the SYTL4–CXCL8 axis thus represents a dual challenge: it not only contributes to tumor growth but also creates an environment conducive to immune suppression.
The implications of these findings are significant. They suggest that by targeting the SLC6A14 pathway, it may be possible to enhance the susceptibility of pancreatic cancer cells to gemcitabine and possibly other chemotherapeutic agents. Such a strategy could pave the way for combination therapies that improve overall survival rates. The researchers advocate for further studies focusing on small molecule inhibitors or monoclonal antibodies that can disrupt SLC6A14 function and subsequently downregulate the SYTL4–CXCL8 axis.
The findings of Kang et al. also raise stimulating questions regarding the metabolic adaptations of cancer cells. As cancer cells frequently rewire their metabolism to support rapid growth, the role of amino acids, particularly glutamine, cannot be overstated. Glutamine serves as a critical energy source for cells during periods of stress, such as during chemotherapy. By emphasizing the SLC6A14-mediated glutamine uptake in conferring gemcitabine resistance, the study encourages a broader reevaluation of metabolic pathways in cancer treatment strategies.
Moreover, this research may have ramifications beyond pancreatic cancer. The principles elucidated in this study could be applicable to other cancers that exhibit similar metabolic dependencies and immune evasion mechanisms. As the landscape of cancer research continues to evolve, understanding the unique tumor microenvironment and the molecular pathways that cancers exploit will be paramount in developing future therapies.
The collaborative approach taken by the researchers illustrates the necessity of interdisciplinary efforts in cancer research. By examining the interplay between metabolic pathways and immune responses, this study exemplifies how innovative perspectives can yield valuable insights into cancer biology. Future research could benefit from similar integrative models that combine metabolic profiling with immunological analyses, offering a robust framework for understanding complex malignancies.
As we look to the future, the call to action becomes clear: targeting metabolic pathways could be the missing link in reversing chemoresistance in pancreatic cancer. The findings of this study will undoubtedly serve as a catalyst for further exploration and validation, inspiring new therapeutic strategies that could alter the course of treatment for patients battling this devastating disease.
The landscape of pancreatic cancer treatment is on the verge of evolution. With the promising insights revealed by Kang et al., there remains hope that the integration of metabolic targeting with existing therapies can revolutionize treatment protocols. The potential to improve treatment efficacy through a better understanding of the SLC6A14-mediated glutamine and SYTL4–CXCL8 axis dynamics marks a significant step forward in cancer research, raising the prospect of bespoke medical interventions tailored to the metabolic needs of individual tumors.
As these new findings circulate through the medical community, the anticipation for future clinical trials aimed at validating the role of SLC6A14 continues to grow. Patients, oncologists, and researchers alike are watching closely, hopeful for the advancements that could stem from this vital connection between metabolism and cancer resistance. The road ahead may be long, but the collective efforts being made today will undoubtedly yield tomorrow’s breakthroughs.
Ultimately, the significance of this work lies not only in its immediate findings but also in its potential to inspire a new generation of targeted therapies in oncology. The research presented by Kang et al. echoes a resounding message: understanding the metabolic underpinnings of cancer can be a game-changer in our approach to treatment, especially in diseases as formidable as pancreatic cancer.
In conclusion, the study conducted by Kang and associates is a powerful reminder that the microscopic intricacies of cellular behavior hold profound implications for the macroscopic challenges faced by the medical community. With continued investigation and clinical application, we may soon witness a transformative shift in the treatment paradigm for pancreatic cancer and beyond, effectively addressing the dual challenges of drug resistance and immune evasion.
Subject of Research: Pancreatic Cancer Chemotherapy Resistance
Article Title: SLC6A14-mediated glutamine promotes SYTL4–CXCL8 axis activation to drive gemcitabine resistance and immune evasion in pancreatic cancer.
Article References: Kang, H.W., Kim, J.H., Jeong, J.W. et al. SLC6A14-mediated glutamine promotes SYTL4–CXCL8 axis activation to drive gemcitabine resistance and immune evasion in pancreatic cancer. Exp Mol Med 57, 2943–2956 (2025). https://doi.org/10.1038/s12276-025-01596-w
Image Credits: AI Generated
DOI: 25 December 2025
Keywords: Pancreatic Cancer, SLC6A14, Gemcitabine, SYTL4, CXCL8, Immune Evasion, Chemoresistance, Glutamine Metabolism.
Tags: amino acid transport in malignanciesbiochemical pathways in cancer resistancechemoresistance in pancreatic cancer therapygemcitabine resistance mechanismsGlutamine metabolism in pancreatic cancerimmune evasion in pancreatic tumorsmolecular interactions in drug resistancepancreatic cancer treatment challengesresearch findings on pancreatic cancer therapySLC6A14 protein role in cancerSYTL4–CXCL8 axis activationtherapeutic strategies for pancreatic cancer