In a groundbreaking advancement in the fight against climate change, researchers from Harvard University’s John A. Paulson School of Engineering and Applied Sciences (SEAS) have unveiled a novel approach to carbon capture using organic molecules known as quinones. This method poses a promising alternative to traditional carbon capture technologies, specifically with respect to their efficacy and environmental friendliness. Traditional methods such as amine scrubbing present significant challenges due to their dependence on substantial energy inputs and the use of corrosive materials, raising concerns about their sustainability and long-term viability.
The newly developed quinone-mediated electrochemical carbon capture systems operate in aqueous solutions, utilizing the unique properties of quinones to selectively bind to carbon dioxide (CO2) molecules. The recent publication of this research in Nature Chemical Engineering highlights the intricate mechanisms underpinning this novel method and emphasizes its potential flat against the backdrop of an escalating climate crisis. Understanding these mechanisms is vital for the optimization and refinement of carbon capture technologies, which need to scale up for industrial applications effectively.
At the helm of this research is Kiana Amini, a former postdoctoral fellow at Harvard, now positioned as an assistant professor at the University of British Columbia. Amini’s contributions are significant—not only does she lead the research, but she also emphasizes the importance of dissecting the various components and contributions to the overall efficacy of the systems she studies. Within this context, the method devised by Amini and her collaborators marks a significant leap forward, offering insights that were previously obscured, likening the electrochemical device to a “black box,” the contents of which were largely unknown.
This study meticulously outlines two critical pathways through which quinones capture carbon—a process that simultaneously occurs yet was previously not fully understood. The first mechanism is a direct carbon capture method, wherein quinones undergo a reduction reaction upon receiving an electrical charge. This reaction enhances their affinity for CO2, allowing them to chemically bond with the gas to form quinone-CO2 adducts, which are a pivotal part of the capture process.
The second mechanism focuses on indirect capture. In this process, charged quinones interact with protons in the solution, contributing to an increase in pH levels. This favorable environment enables CO2 to react more readily with water, resulting in the formation of bicarbonate or carbonate compounds. Amini elucidates that identifying the specific contributions of these two mechanisms is essential for optimizing the performance of quinone-mediated carbon capture systems, making it an important area of focus in ongoing research.
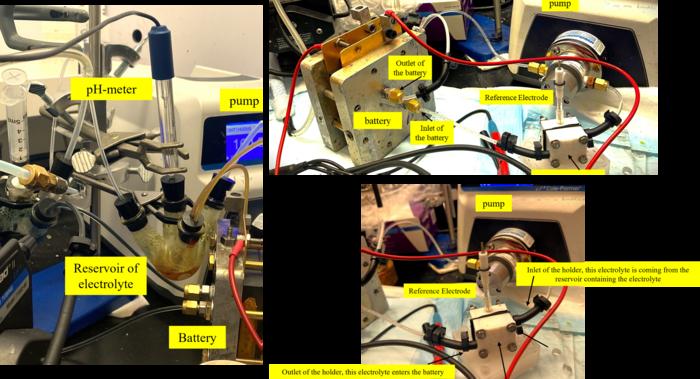
Employing innovative experimental methodologies, the researchers were able to quantify both capture mechanisms in real-time. The first method involved the use of reference electrodes to measure the voltage differentials arising from the quinones, as well as the resultant quinone-CO2 adducts. This approach allowed the team to observe fluctuations in electrical activity that correlate with carbon capture dynamics, thereby yielding quantitative insights into the processes at play.
Additionally, the researchers utilized fluorescence microscopy to differentiate among varying chemical states of the quinones. By analyzing the fluorescence signatures unique to the chemicals involved in the process, they were able to detect and quantify the concentrations of oxidized, reduced, and adduct species at rapid time intervals. Harnessing such techniques allows for a deeper understanding of the mechanisms and enables them to model and improve the reaction kinetics effectively.
The transformative nature of this research expands the horizons of aqueous quinone-based carbon capture systems, equipping scientists with the necessary tools to tailor designs for specific industrial applications. Indeed, adapting these systems for real-world industrial contexts is a crucial goal. While the study offers robust insights into the operational mechanisms, researchers are also cognizant of challenges that lie ahead. Notably, the tendency of these systems to be oxygen-sensitive may hinder performance and further investigations will be required to address this issue.
The implications of this research stretch beyond mere academic interest. With the alarming increase in atmospheric CO2 levels, finding effective ways to capture and reduce carbon emissions has become increasingly imperative. If the mechanisms elucidated through this study are harnessed effectively, they point toward a future where carbon capture systems can not only mitigate pollution but also integrate harmoniously into existing industrial frameworks.
As global leaders and policymakers increasingly prioritize carbon neutrality and sustainability in their agendas, innovations such as quinone-mediated carbon capture could very well play a pivotal role in achieving these targets. Furthermore, as the technology matures, it may contribute significantly to the development of more efficient and sustainable energy systems, thereby reshaping societal approaches to energy generation and consumption.
The work undertaken by Amini and her team stands as a testament to the significance of interdisciplinary research in tackling environmental challenges. By combining principles from chemistry, engineering, and materials science, they are paving the way for the next generation of carbon capture technologies. This study not only enriches the scientific community’s understanding of quinone chemistry but also lays the groundwork for real-world applications that could impact numerous industries, from energy to manufacturing.
Ultimately, as efforts continue to combat climate change, the innovative strategies and methodologies discovered in this research serve as a beacon of hope. By highlighting the importance of understanding chemical interactions at the molecular level, this work inspires future research and development across diverse fields. The art of refining carbon capture methods will require sustained efforts, creativity, and collaboration, but the potential rewards—both for the planet and future generations—are incalculable.
Subject of Research: Quinone-mediated electrochemical carbon capture
Article Title: In situ techniques for aqueous quinone-mediated electrochemical carbon capture and release
News Publication Date: 16-Dec-2024
Web References: Nature Chemical Engineering
References: DOI: 10.1038/s44286-024-00153-y
Image Credits: Kiana Amini / UBC
Keywords
Carbon capture, Electrochemical systems, Quinones, Climate change mitigation, Industrial applications, Aqueous systems, Green chemistry, Sustainability innovations, CO2 adducts, Chemical engineering.