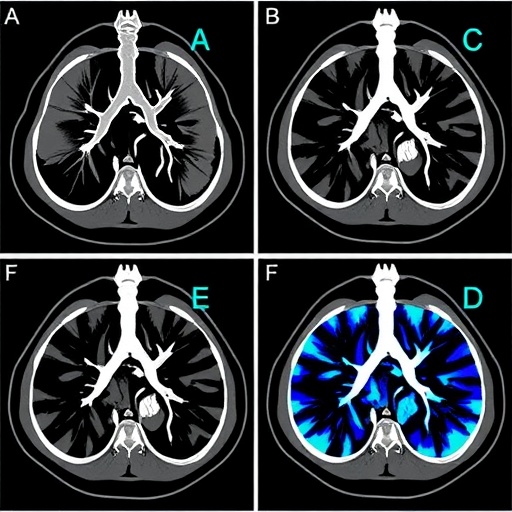
In the ever-evolving landscape of oncological imaging, researchers have recently made a pivotal stride in improving the early diagnosis of lung adenocarcinoma (LUAD), particularly in its nascent clinical stage IA classification. A newly published study in the Journal of Thoracic Disease presents compelling evidence demonstrating that the fluorine-18 labeled fibroblast activation protein inhibitor (^18F-FAPI-04) in positron emission tomography/computed tomography (PET/CT) outperforms the long-standing ^18F-fluorodeoxyglucose (^18F-FDG) tracer in detecting early-stage LUAD lesions. This breakthrough holds significant promise for refining diagnostic accuracy and, by extension, enhancing patient prognoses by enabling more timely therapeutic interventions.
Historically, ^18F-FDG PET/CT has been the cornerstone imaging modality in the staging and restaging of various lung cancers, capitalizing on the heightened glycolytic activity characteristic of malignant cells. However, this metabolic imaging approach exhibits limitations, particularly when confronting tumors less than 1.0 cm in diameter, as well as histologically subtle entities such as adenocarcinoma in situ and minimally invasive adenocarcinoma. These sub-centimeter lesions often evade accurate detection due to their lower glucose metabolism and the spatial resolution thresholds of conventional PET scans, precipitating diagnostic ambiguity and potentially delaying crucial clinical decisions.
Addressing these constraints, the investigation in question undertook a methodical comparison between ^18F-FAPI-04 and ^18F-FDG PET/CT in a cohort of patients with stage IA LUAD. The choice of ^18F-FAPI-04 is scientifically grounded in its mechanism of targeting the fibroblast activation protein (FAP), a serine protease selectively overexpressed in cancer-associated fibroblasts (CAFs) within the tumor microenvironment. CAFs are increasingly recognized as pivotal facilitators of tumor progression, immune evasion, and metastatic dissemination. By imaging FAP expression, ^18F-FAPI-04 PET/CT offers an indirect, yet highly specific, biomarker-based visualization of tumor-associated stromal activity, complementing or potentially superseding the metabolic focus of ^18F-FDG imaging.
Quantitative analyses from the study revealed a statistically significant elevation in the maximum standardized uptake value (SUVmax) and the tumor-to-background ratio for ^18F-FAPI-04 compared with ^18F-FDG in stage IA LUAD lesions. The SUVmax is a critical metric reflecting tracer accumulation intensity, serving as a proxy for tumor biological activity. Enhanced tumor-to-background contrast with ^18F-FAPI-04 indicates superior lesion conspicuity, which directly influences diagnostic confidence and the accuracy of tumor delineation during clinical assessment.
The correlation between ^18F-FAPI-04 uptake and ex vivo immunohistochemical detection of FAP expression in resected tumor specimens furnishes robust validation for the imaging modality’s specificity. This pathological confirmation anchors the molecular imaging findings to tangible biological phenomena within the tumor microenvironment, reinforcing the tracer’s role as a bona fide marker of stromal activation rather than nonspecific uptake.
Methodologically, the study was observational in design, encompassing patients clinically staged as IA LUAD who underwent parallel ^18F-FDG and ^18F-FAPI-04 PET/CT examinations prior to surgical resection. Postoperative pathological evaluation facilitated both histopathological confirmation and FAP immunostaining, enabling a comprehensive cross-validation approach. This design enhances translational validity, bridging radiological, molecular, and pathological domains.
The implications of these findings are multifaceted. Clinically, the adoption of ^18F-FAPI-04 PET/CT could revolutionize the diagnostic algorithm for early-stage lung adenocarcinoma, enabling the detection of lesions that conventional ^18F-FDG imaging may overlook. Earlier and more accurate detection has profound therapeutic ramifications, potentially allowing for less invasive interventions and tailored treatment strategies that improve patient survival rates.
Moreover, the study highlights the evolving recognition of the tumor microenvironment as a dynamic participant in oncogenesis and cancer progression. Imaging fibroblast activation protein expression underscores a paradigm shift toward stromal targeting, expanding the scope of molecular imaging beyond tumor cells alone. This approach not only enriches diagnostic precision but may also open avenues for FAP-targeted therapeutics and theranostic applications.
In technical terms, the higher specificity and affinity of ^18F-FAPI-04 for activated fibroblasts overexpressing FAP enhances tumor visualization by reducing background noise in surrounding tissues. Unlike ^18F-FDG, which accumulates nonspecifically in inflammatory or metabolically active benign tissues, ^18F-FAPI-04 promises a more tumor-selective imaging profile, mitigating false-positive results that complicate clinical interpretation.
The study’s statistical rigor, evident in the achievement of significance thresholds (P < 0.05), and its use of gold standard histopathology for validation confers high scientific credibility. Its findings resonate with emerging literature emphasizing the clinical and biological merits of FAP-targeted imaging agents in diverse solid tumors, affirming the broader applicability of such novel tracers.
Future research trajectories may explore longitudinal assessments of ^18F-FAPI-04 PET/CT in monitoring therapeutic response, detection of recurrence, and integration into multimodal imaging protocols. Additionally, the safety profile and dosimetry parameters of ^18F-FAPI-04 warrant continued scrutiny to ensure clinical best practices and patient safety.
The funding sources backing this work, including the Special Fund Project of Science and Technology in Maoming Guangdong China and the Guangdong Medical Research Fund, underscore continued institutional support for innovative cancer imaging research. Furthermore, the authors’ adherence to conflict-of-interest transparency, with no declared competing interests, reinforces the work’s integrity.
Collectively, this study exemplifies the convergence of molecular biology, radiochemistry, and clinical practice, offering a poignant example of how targeted molecular imaging can refine early cancer detection. The evolution from conventional metabolic imaging to stromal-targeted diagnostics heralds an exciting era where the tumor microenvironment is leveraged for both diagnostic and therapeutic gain. For patients at the earliest stages of lung adenocarcinoma, these advancements represent not merely improvements in imaging techniques but potentially vital steps toward improved survival and quality of life.
Subject of Research: People
Article Title: Comparison of the diagnostic accuracy between 18F-FAPI-04 PET/CT and 18F-FDG PET/CT in the clinical stage IA of lung adenocarcinoma
News Publication Date: 27-Feb-2025
Web References: http://dx.doi.org/10.21037/jtd-24-1658
References: Liang HX, Huang QW, He YM, Mai YQ, Chen ZL, Wang BP, Fang N, Hu JF, Li X, Zhang N, Liu ET, Li XC. Comparison of the diagnostic accuracy between 18F-FAPI-04 PET/CT and 18F-FDG PET/CT in the clinical stage IA of lung adenocarcinoma. J Thorac Dis 2025;17(2):661-675. doi: 10.21037/jtd-24-1658
Keywords: Respiratory disorders, lung adenocarcinoma, PET/CT imaging, 18F-FAPI-04, 18F-FDG, fibroblast activation protein, tumor microenvironment, early cancer detection
Tags: 18F-FAPI-04 PET/CT imaging18F-FDG PET/CT limitationsclinical stage IA lung adenocarcinomadiagnostic accuracy in lung adenocarcinomaearly diagnosis of lung cancerenhancing therapeutic interventions in oncologyfibroblast activation protein inhibitorimproving patient prognoses in cancermetabolic imaging techniquesoncological imaging advancementsPET/CT comparison studiessub-centimeter lung lesions detection