Discovery of a mechanism that produces reactive oxygen species and invites cellular senescence
Credit: Kobe University
Permanently arrested cell growth is known as “cellular senescence”, and the accumulation of senescent cells may be one cause of aging in our bodies. Japanese researchers have discovered that a certain enzyme in our bodies promotes cellular senescence by producing reactive oxygen species. Drugs that target this enzyme could potentially suppress this process, and inhibit aging and aging-related illnesses.
This discovery was made by a Kobe University research team led by Professor Shinji Kamada and Research Fellow Taiki Nagano. The findings were published on January 18 in Life Science Alliance, a new open access journal created by a partnership between EMBO Press, Rockefeller University Press and Cold Spring Harbor Laboratory Press.
Staying healthy in old age is a pressing issue, particularly in “super-aging” societies such as Japan. The relationship between aging and cellular senescence is still not clear, but experiments using mouse models have found that removing senescent cells inhibits aging and aging-related illnesses.
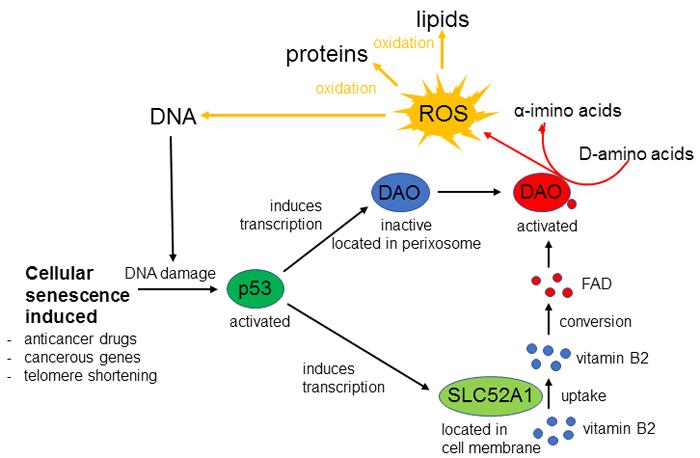
Discovered about 80 years ago, D-amino acid oxidase (DAO) is an enzyme that catalyzes the oxidative deamination of D-amino acids. The research team induced cellular senescence by treating cancerous cells with low concentrations of anticancer drugs and causing DNA double-strand breaks. They discovered that the increased expression of DAO is dependent on a transcription factor known as p53, which has cancer-suppressing properties. Increased expression of DAO was confirmed in senescent cells induced by various methods, proving that DAO plays an important role in regulating cell senescence.
DAO oxidizes D-amino acids to produce α-imino acids, also producing reactive oxygen species (ROS) as a by-product. The accumulation of ROS has been linked both to aging and cellular senescence, so the team looked at the relationship between DAO and ROS in causing cellular senescence.
When DAO was knocked down and its activity suppressed, cellular senescence and ROS production were inhibited. The team also found that a DAO mutant without enzymatic properties did not promote cellular senescence or produce ROS. In other words, DAO facilitates cellular senescence by producing ROS through an enzymatic reaction.
The activation of cellular senescence by DAO was only observed when anticancer drugs induced a DNA double-strand break. The team predicted that a gene induced by DNA damage is contributing to this process. They found that when DNA is damaged by shortening of telomeres, treatment by anticancer drugs or the activation of cancer genes, transcription is induced in DAO and p53-regulated genes including the transporter SLC52A1. DAO is localized within the cell peroxisome, but its activity is low because there are insufficient amounts of co-enzyme flavin adenine dinucleotide (FAD). SLC52A1 brings in vitamin B2 from outside the cell, this is converted to FAD in the cell, and when FAD concentration increases DAO is activated. ROS, the by-product of the reaction, damage cells by oxidizing DNA, proteins and lipids, which promotes cellular senescence (figure 1).
As implied by their name, ROS are very reactive, and it is thought that they play a part in causing diseases such as Alzheimers, Parkinsons, cancers and diabetes, as well as aging. However, ROS also work beneficially in our bodies. Low concentrations of ROS increase our lifespans, and ROS are used when our immune systems eliminate infecting organisms. It is clear that when ROS are overproduced they have a negative effect, and ROS produced under stress in particular are thought to cause illness and aging. This study has revealed a mechanism for ROS produced when cellular senescence is induced by stress.
###
Media Contact
Eleanor Wyllie
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.




