Scientists explain the role of a certain protein in the generation of cells critical to bone maintenance
Credit: Tokyo University of Science
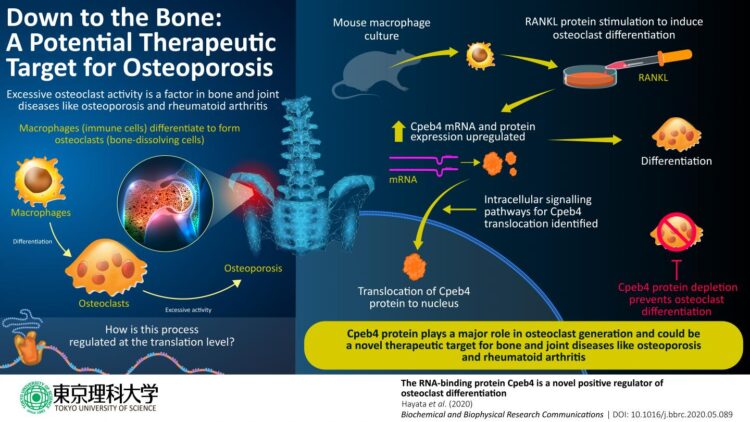
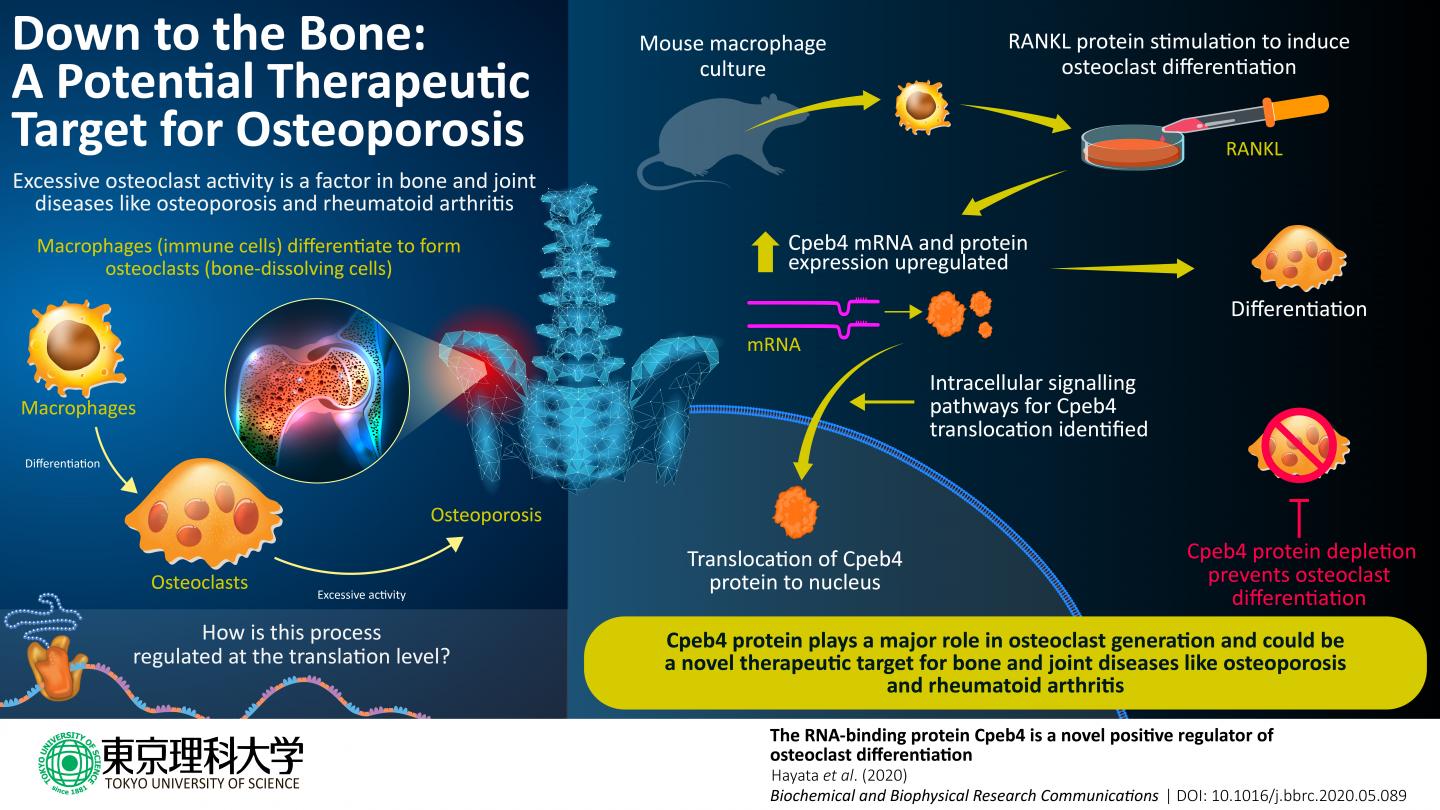
Chronic bone and joint diseases, such as osteoporosis and rheumatoid arthritis, affect millions of people worldwide, particularly the elderly, degrading their quality of life. An important factor in both of these diseases is the excessive activity of bone-dissolving cells called osteoclasts. Osteoclasts are formed through differentiation from a certain type of immune cell called macrophage, after which they acquire their new role in the maintenance of bones and joints: breaking down bone tissue to allow osteoblasts-another type of cell-to repair and remodel the skeletal system.
Broadly, two intracellular processes are involved in this differentiation: first, transcription-in which a messenger RNA (mRNA) is created from the genetic information in DNA-and then, translation-in which the information in the mRNA is decoded to produce proteins that perform specific functions in the cell. Since the discovery of the role of a particular protein called RANKL in osteoclast formation, scientists have solved a considerable portion of the puzzle of which cell signaling pathways and transcription networks regulate osteoclast generation. Yet, the post-transcription cellular processes involved remain to be understood.
Now, in a new study published in Biochemical and Biophysical Research Communications, scientists at Tokyo University of Science, Japan, unraveled the role of a protein called Cpeb4 in this complex process. Cpeb4 is part of the “cytoplasmic polyadenylation element binding (CPEB)” family of proteins, which bind to RNA and regulate translational activation and repression, as well as “alternative splicing” mechanisms that produce protein variants. Dr Tadayoshi Hayata, who led the study, explains: “CPEB proteins are implicated in various biological processes and diseases, such as autism, cancer, and red blood cell differentiation. However, their functions in osteoclast differentiation are not clearly known. Therefore, we conducted a series of experiments to characterize a protein from this family, Cpeb4, using cell cultures of mouse macrophages.”
In the various cell culture experiments conducted, mouse macrophages were stimulated with RANKL to trigger osteoclast differentiation and the evolution of the culture was monitored. First, the scientists found that Cpeb4 gene expression, and consequently the amount of Cpeb4 protein, increased during osteoclast differentiation. Then, through immunofluorescence microscopy, they visualized the changes in the location of Cpeb4 within the cells. They found that Cpeb4 moves from the cytoplasm into nuclei, while presenting specific shapes (osteoclasts tend to fuse together and form cells with multiple nuclei). This indicates that the function of Cpeb4 associated with osteoclast differentiation is likely carried out inside the nuclei.
To understand how RANKL stimulation causes this Cpeb4 relocalization, the scientists selectively “inhibited” or represses some of the proteins that become involved “downstream” in the intracellular signaling pathways triggered by the stimulation. They identified two pathways as necessary for the process. Nonetheless, further experiments will be required to fully learn about the sequence of events that takes place and all the proteins involved.
Finally, Dr Hayata and his team demonstrated that Cpeb4 is absolutely necessary for osteoclast formation using macrophage cultures in which Cpeb4 was actively depleted. The cells in these cultures did not undergo further differentiation to become osteoclasts.
Taken together, the results are a stepping stone to understanding the cellular mechanisms involved in osteoclast formation. Dr Hayata remarks: “Our study sheds light on the important role of the RNA-binding protein Cpeb4 as a positive “influencer” of osteoclast differentiation. This gives us a better understanding of the pathological conditions of bone and joint diseases and may contribute to the development of therapeutic strategies for major diseases like osteoporosis and rheumatoid arthritis.” Hopefully, the deeper level of understanding of osteoclast generation facilitated by this study will ultimately translate into improved quality of life for people living with painful bone and joint diseases.
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized
private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido.
Established in 1881, the university has continually contributed to Japan’s development in science through
inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and
society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a
multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a
meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that
has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the
natural sciences field.
Website: https:/
About Associate Professor Tadayoshi Hayata from the Tokyo University of Science
Since 2018, Dr Tadayoshi Hayata has been Associate Professor and Principal Investigator at the Department of Molecular Pharmacology, Faculty of Pharmaceutical Science, at the Tokyo University of Science. His laboratory focuses on bone metabolism, cellular differentiation, molecular pharmacology, and similar fields to understand the nature of bone and joint diseases and find therapeutic targets. Dr Hayata is affiliated with several Japanese Societies and the American Society for Bone and Mineral Research. He has published over 50 original articles and given over 150 presentations at academic conferences. In addition, his research on osteoporosis has made it to Japanese newspapers several times.
https:/
Media Contact
Tsutomu Shimizu
[email protected]
Original Source
https:/
Related Journal Article
http://dx.