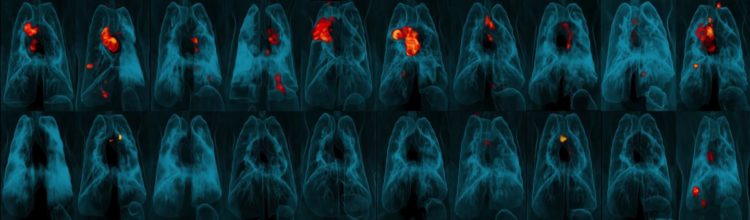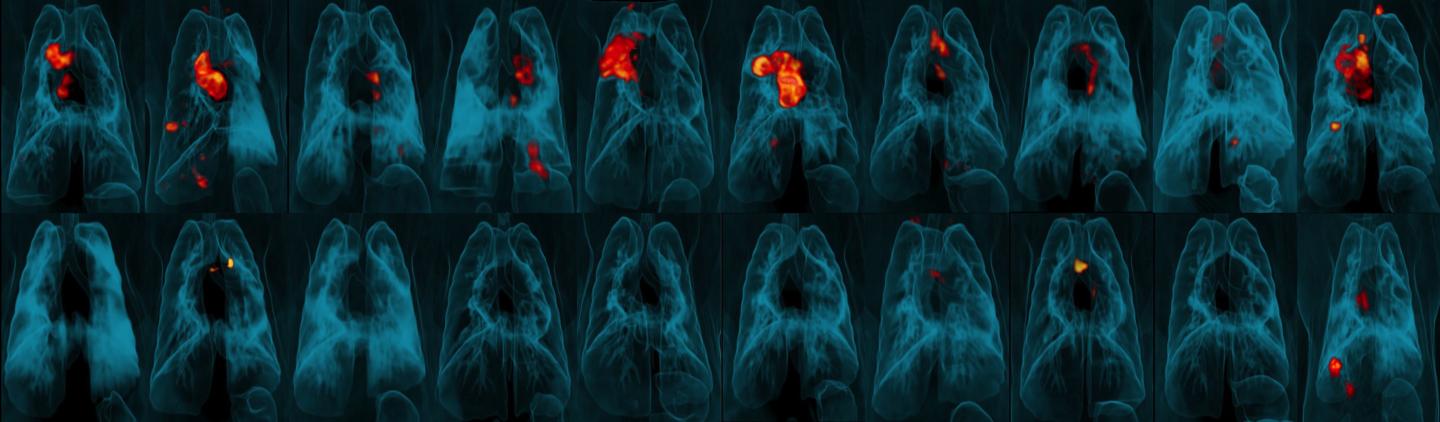
Credit: JoAnne Flynn, Ph.D., Alexander White, Pauline Maiello, and Mario Roederer, Ph.D.
PITTSBURGH, Jan. 1, 2020 – Worldwide, more people die from tuberculosis (TB) than any other infectious disease, even though the vast majority were vaccinated. The vaccine just isn’t that reliable. But a new Nature study finds that simply changing the way the vaccine is administered could dramatically boost its protective power.
Researchers at the University of Pittsburgh School of Medicine and the National Institute of Allergy and Infectious Diseases (NIAID) discovered that intravenous TB vaccination is highly protective against the infection in monkeys, compared to the standard injection directly into the skin, which offers minimal protection.
“The effects are amazing,” said senior author JoAnne Flynn, Ph.D., professor of microbiology and molecular genetics at the Pitt Center for Vaccine Research. “When we compared the lungs of animals given the vaccine intravenously versus the standard route, we saw a 100,000-fold reduction in bacterial burden. Nine out of 10 animals showed no inflammation in their lungs.”
Flynn’s team tested several routes and doses of the only commercially available human TB vaccine, Bacille Calmette-Guérin (BCG), which is made of a live, weakened form of TB bacteria found in cattle.
The BCG vaccine has been around for 100 years and is among the most widely used vaccines in the world, but its efficacy varies widely.
The idea for an intravenous TB vaccination came from earlier experiments by the other senior author on the study, Robert Seder, M.D., at the NIAID’s Vaccine Research Center. Seder showed in both animals and humans that the malaria vaccine is more effective when delivered intravenously.
To test whether the method of administration matters for TB, Flynn and colleagues separated their colony of monkeys into six groups: unvaccinated, standard human injection, stronger dose but same injection route, mist, injection plus mist, and finally, the stronger dose of BCG delivered as a single shot directly into the vein.
Six months later, the researchers exposed the animals to TB and monitored them for signs of infection.
Monkeys are extremely susceptible to TB. All of the animals who received the standard human dose had persistent lung inflammation, and the average amount of TB bacteria in their lungs was only slightly less than in the monkeys who received no vaccine at all. The other injected and inhaled vaccines offered similarly modest TB protection.
The intravenous vaccine, on the other hand, offered nearly full protection. There was virtually no TB bacteria in the lungs of these animals, and only one monkey in this group developed lung inflammation.
“The reason the intravenous route is so effective,” Flynn explained, “is that the vaccine travels quickly through the bloodstream to the lungs, the lymph nodes and the spleen, and it primes the T cells before it gets killed.”
Flynn’s team found BCG and activated T cells in the lungs of all the intravenously vaccinated animals. Among the other groups, BCG was undetectable in the lung tissue and T cell responses were relatively meagre.
Next, the researchers plan to test whether lower doses of intravenous BCG could offer the same level of protection without the side effects, which mostly consist of temporary inflammation in the lungs.
But before this method can be translated to humans, researchers need to know that it’s not only safe, but also practical. An intravenous vaccine requires more skill to administer and carries a higher risk of infection.
“We’re a long way from realizing the translational potential of this work,” Flynn said. “But eventually we do hope to test in humans.”
###
Additional authors on the study include Patricia Darrah, Ph.D., Joshua Hackney, Supriya Pokkali, Ph.D., Phillip Swanson, M.D., Megha Kamath, and Mario Roederer, Ph.D., of NIAID; Joseph Zeppa, Ph.D., Pauline Maiello, Nicole Grant, Mark Rodgers, M.S., Chelsea Causgrove, Edwin Klein, D.V.M., Alexander White, and Charles Scanga, Ph.D., of Pitt; Marc Wadsworth, Travis Hughes, M.P.H., and Alex Shalek, Ph.D., of the Massachusetts Institute of Technology and Harvard University; Dominick Laddy, Ph.D., and Danilo Casimiro, Ph.D., of Aeras; Aurelio Bonavia, Ph.D., of Vir Biotechnology; and Philana Ling Lin, M.D., of UPMC Children’s Hospital of Pittsburgh.
This research was supported by the Bill and Melinda Gates Foundation, the National Institutes of Health (grants 5U24AI118672 and 2RM1HG006193) and the NIAID Intramural Research Program. PARI Pharma GbmH provided the aerosol vaccine delivery device.
To read this release online or share it, visit https:/
About the University of Pittsburgh School of Medicine
As one of the nation’s leading academic centers for biomedical research, the University of Pittsburgh School of Medicine integrates advanced technology with basic science across a broad range of disciplines in a continuous quest to harness the power of new knowledge and improve the human condition. Driven mainly by the School of Medicine and its affiliates, Pitt has ranked among the top 10 recipients of funding from the National Institutes of Health since 1998. In rankings recently released by the National Science Foundation, Pitt ranked fifth among all American universities in total federal science and engineering research and development support.
Likewise, the School of Medicine is equally committed to advancing the quality and strength of its medical and graduate education programs, for which it is recognized as an innovative leader, and to training highly skilled, compassionate clinicians and creative scientists well-equipped to engage in world-class research. The School of Medicine is the academic partner of UPMC, which has collaborated with the University to raise the standard of medical excellence in Pittsburgh and to position health care as a driving force behind the region’s economy. For more information about the School of Medicine, see http://www.
http://www.
Contact: Erin Hare
Office: 412-864-7194
Mobile: 412-738-1097
E-mail: [email protected]
Contact: Allison Hydzik
Office: 412-647-9975
Mobile: 412-559-2431
E-mail: [email protected]
Media Contact
Erin Hare
[email protected]
412-864-7194
Related Journal Article
http://dx.