Credit: POSTECH
Alzheimer’s disease – also called dementia – where memory and cognitive functions gradually decline due to deformation and death of neurons, and Parkinson’s disease that causes tremors in hands and arms impeding normal movement are major neurodegenerative diseases. Recently, a research team at POSTECH has identified the structure of the agent that causes Alzheimer’s and Parkinson’s diseases to occur together.
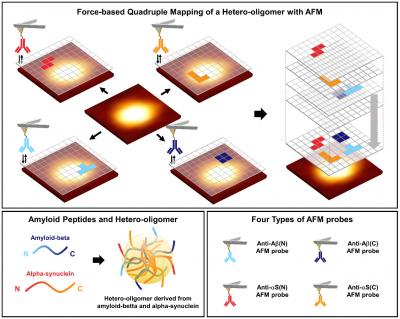
A research team led by Professor Joon Won Park and Ph.D. candidate Eun Ji Shin of the Department of Chemistry at POSTECH investigated the surface structure of hetero-oligomers found in the overlap of Alzheimer’s disease and Parkinson’s disease, using an atomic force microscopy (AFM) to reveal their structural identity. This study was featured as the front cover paper in the latest issue of Nano Letters.
It is known that the pathological overlap of Alzheimer’s disease and Parkinson’s disease is associated with the formation of hetero-oligomers derived from amyloid-beta and alpha-synuclein. However, it was difficult to study the treatment due to technical limitations in observing their structure.
To this, the researchers used the AFM to observe the surface characteristic of the hetero-oligomer nano-aggregates derived from amyloid-beta, known as the biomarker of Alzheimer’s disease, and alpha-synuclein, known as the biomarker of Parkinson’s disease, at the single-molecule level.
When the research team investigated with four AFM tips immobilized with antibodies that recognize N-terminus or C-terminus of each peptide, it was confirmed that all aggregates were hetero-oligomers. In addition, in the case of hetero-oligomer, it was confirmed that the probability of recognizing the end of the peptide is higher than that of the homo-oligomer.
This result indicates that the end of each peptide has a bigger tendency to be located on the surface of hetero-oligomers than homo-oligomers, or that the ends of the peptides located on the surface have more degrees of freedom. That is, it can be confirmed that the aggregation between peptides is more loosely packed in the hetero-oligomer than in the homo-oligomer.
This study is the first study to observe the structure of protein disordered nano-aggregates, which has never been identified before, using the quadruple mapping with four AFM tips. It serves as experimental grounds to verify the hypothesis of hetero-oligomer aggregation. It can also be used in studies related to the overlapping phenomena of various neurodegenerative diseases other than Alzheimer’s and Parkinson’s.
“Until now, there was no adequate method to analyze the nano-aggregates, making it impossible to elucidate the structural identity of heterogeneous aggregates,” explained Professor Joon Won Park. “As the analysis method developed in this study is applicable to other amyloid protein aggregates, it will help to identify the cause of diseases such as Alzheimer’s or the mad cow disease.”
###
This study was conducted with the support from the Mid-career Researcher Program and the Global Ph.D. Fellowship Program of the National Research Foundation of Korea.
Media Contact
Jinyoung Huh
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




