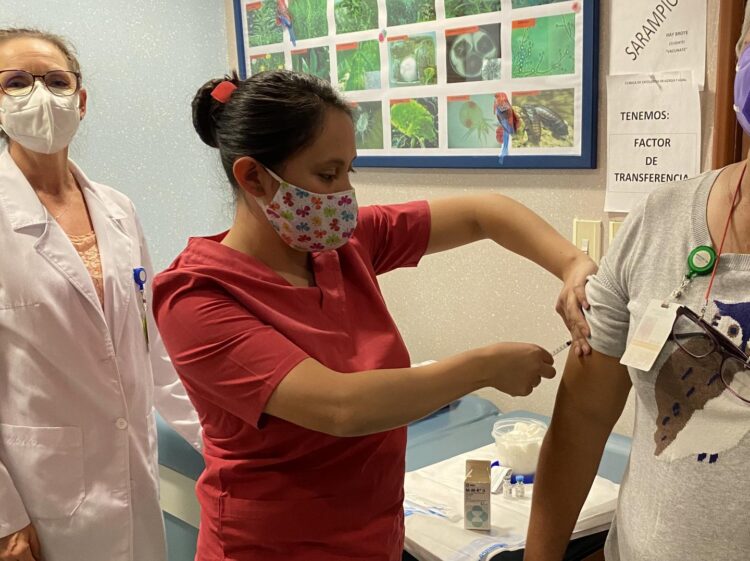
A prospective observational study testing the concept of trained immunity for protection from severe COVID-19 reported milder symptoms in COVID patients receiving MMR vaccination, as published in Allergy.
Credit: Centro de Excelencia en Asma y Alergia
COVID-19 continues to wreak havoc globally, with over one million deaths to date. Yet what if an existing vaccine could make COVID-19 less deadly? A study just published put the theory to test, with promising results.
A research team led by Dr. Larenas-Linnemann working at Medica Sur, Mexico City, reported on their clinical observations in 255 subjects vaccinated with the mumps-measles-rubella (MMR) vaccine since the start of the Coronavirus disease-2019 (COVID-19) pandemic. Many vaccinated patients were family members or caregivers of patients who already had contracted COVID-19, and thus at extremely high risk. Thirty-six of the patients have now contracted COVID-19, but all with a remarkably mild course, with less severe symptoms than would be expected given their health status and age. The paper, published in the September issue of Allergy, the European Journal of Allergy and Immunology, is now available for free download.
MMR vaccination in the context of COVID-19, taking advantage of a measles outbreak
At the start of the COVID-19 pandemic, observing the highly contagious and virulent nature of the virus and the lack of available preventive measures, the investigators searched for methods to enhance innate immunity — in effect, give the immune system a boost to prepare it for a variety of assaults. Since COVID-19 was new to humans, there was no existing treatment or vaccine to specifically fight it. But Dr. Larenas-Linnemann, being Dutch and having done her basic training in the Netherlands, had followed with admiration the work of a fellow countryman on a little-known concept called “trained immunity.” In technical terms, trained immunity refers to the enhanced immune response to a certain pathogen, after being exposed (by vaccination or natural illness) to another non-related pathogen; the immune reaction after a subsequent exposure to a non-related pathogen is faster in onset and accompanied by an increased production of certain cytokines. This means that, surprisingly, some vaccinations could not only prevent the target disease (such as measles), but also help people fight other diseases.
Trained immunity is a form of non-specific immunity. It was controversial at first, but after decades of pioneering fieldwork from a Danish team studying the tuberculosis vaccine and the live polio vaccine in both Northern Europe and Africa, followed by years of authoritative laboratory work from Dr. Larenas-Linnemann’s countryman Mihai Netea and others, it is now accepted that certain live-attenuated vaccines can make the body better prepared to fight off a range of pathogens.
Dr. Larenas-Linnemann’s team wondered: Could a live-attenuated vaccine be protective against this entirely new disease, COVID-19, for their patients? Taking advantage of the fact that the pandemic in Mexico coincided with a rise in measles cases, which had motivated the Ministry of Health to recommend measles re-vaccination, the team decided to put the concept of trained immunity to the test. As such, from March 2020 onward, the researchers recommended MMR vaccination to their patients, especially among family members of COVID-19 cases.
Observational trial of MMR for COVID-19 prevention
In a prospective observational trial in Hospital Médica Sur, ranked since 2011 as the best hospital of Mexico City, the team gave their patients boosters of a standard childhood vaccine, measles-mumps-rubella (MMR), which is considered safe worldwide (a booster is even required in many places to start college or military service). The patients were followed closely to watch for COVID-19 infection. The patients were considered high risk for COVID infection because many were caring for ill family members and were vulnerable due to their age or other risk factors.
COVID-19 infection was considered confirmed with a positive result of a SARS-CoV-2 antigen test, the detection of specific antibodies, or the combined presence of a direct contact with a confirmed case plus anosmia/ageusia and at least two classic COVID-19 symptoms. Direct contact with a confirmed case, accompanied by classic symptoms, but without smell or taste changes were considered highly probable cases. The clinical severity of COVID-19 was graded on a simplified scale from zero for asymptomatic cases, through 1 for mild, 2 for moderate, 3 for lower respiratory symptoms without the need for oxygen, up to 6 for fatality. Also, home measurements of pulse oximetry and peak-expiratory-flow rate (PEFR) were used to determine severity.
Outcomes: none of the 36 cases needed supplementary oxygen
Among the 255 vaccinated subjects, there were 24 confirmed and 12 (highly) probable COVID-19 cases, thirteen with risk factors that can often make COVID-19 more serious (hypertension, diabetes, obesity, smoking, or uncontrolled asthma). In their publication, the authors detail the risk factors for each patient. All received general supportive measures; some received off-label high-dose ivermectin the first two days. In general, the cases were less severe than would be expected. All had minor respiratory symptoms at most. Only one patient, with uncontrolled asthma, had one day of mild low blood oxygen. No patients had respiratory insufficiency to the degree of needing oxygen.
“We were relieved that MMR, which is commonly thought of as a childhood vaccination, seemed to help our older adult patients weather the storm too,” said Dr. Larenas-Linnemann.
Reaction
“We would not be surprised if MMR could provide some protection against severe COVID-19,” said researcher Peter Aaby, of Bandim Health Project in Guinea-Bissau and Research Centre for Vitamins and Vaccines (CVIVA), Statens Serum Institut, a governmental public health and research institution under the Danish Ministry of Health in Copenhagen, Denmark and a pioneer in the field. “Together with my partner Dr. Christine Stabell Benn, we’ve been reporting on mortality reductions from live-attenuated vaccines such as polio, BCG and measles vaccine/MMR for multiple decades now, and arguing for optimized vaccine schedules. With the COVID-19 crisis adding urgency, it’s good to see the potential of non-specific immune effects being taken seriously.”
Global research applying trained immunity to COVID-19 prevention
Researchers around the world are studying the ability of existing live-attenuated vaccines to prevent severe complications of COVID-19.
Several clinical trials are now ongoing with tuberculosis (BCG) vaccination of SARS-CoV-2 exposed health-care workers to reduce the severity of an eventual infection. However, one of the effects described in experiments with BCG-trained-immunity was a rise in interleukin 6 cytokine (IL-6), which made the Médica Sur team reluctant to use this method due to immune over-activation linked to high IL-6 levels described in severe COVID-19 cases. In addition, BCG vaccine and live polio vaccine are not used in the United States of America or Canada, making work with the BCG and polio vaccines harder to apply widely across North America. Existing supplies of BCG are also critically needed to save infant lives around the globe. Dr. Larenas-Linnemann’s team instead chose the MMR vaccine for its safety profile, as well as for previous research describing trained immunity from MMR in newborns of hepatitis-infected mothers and retrospective studies showing 26-49% decreases in all-cause mortality rates after measles vaccination programs.
What’s next
These first observational data gathered by Dr. Larenas-Linnemann and her group in Mexico City, demonstrating a milder course of COVID-19 in recently MMR-vaccinated individuals, are promising. The investigators are continuing their strategy to recommend MMR vaccination to household contacts of COVID-19 patients and plan to keep collecting data on further clinical cases. They are also searching for cooperation with basic immunologists to study the immunologic background of their observations. Said Dr. Larenas-Linnemann, “We are grateful to be able to have been able to offer this to our patients and hope these first real-life data will spark interest in the approach.”
Conclusive evidence of the value of MMR vaccine to reduce COVID-19 complications requires a prospective, randomized trial. This is exactly what microbiologist Dr. Paul Fidel of Louisiana State University has recently launched. He has hypothesized that live attenuated vaccines induce myeloid-derived suppressor cells (MDSCs), another form of trained innate immunity, to suppress the fatal sepsis often seen in severe COVID-19 cases, as outlined in an opinion/hypothesis piece in mBio. With funding from the Parsemus Foundation and Fast Grants, the MMR randomized controlled trial is enrolling first responders and healthcare workers in the hard-hit New Orleans region. Dr. Fidel’s team is still seeking funding to test whether MMR can provide protection to nursing home residents, who are particularly vulnerable to this disease.
###
About the Center of Excellence in Asthma and Allergy
Clinica en Alergia y Asma was accredited as a Center of Excellence by GA2LEN in May 2016. In March 2017, the clinic was renamed and officially established as the Centro de Excelencia en Asma y Alergia Larenas, and now both conducts research and provides advanced care to adults and children with allergic diseases, including asthma. The Center was recertified as a Center of Excellence in 2019 and is located within the office tower of the Hospital Medica Sur, in Mexico City.
About Medica Sur
Medica Sur is a highly specialized health institution made up of medical, diagnostic, research, teaching and social assistance units. Medica Sur brings together a select group of professionals from medicine, nursing, administration and hospital operations, who – guided by a strict code of ethics and backed by cutting-edge technologies – aim to offer its patients medical excellence with a human touch.
Medica Sur was the first hospital outside the United States to be part of the Mayo Clinic Care Network, thereby promoting collaboration between physicians to improve medical care for all their patients in Mexico.
It is certified by the General Health Council of Mexico (Consejo de Salubridad General), validating the quality and safety in the medical care of its patients and families. It has been certified by Trace International for ensuring that its background and policies meet the highest international ethical and anti-bribery standards. It also has the Accreditation of Joint Commission International (JCI), an international, external and independent body that evaluates the quality and safety of health services; Medica Sur has been accredited since 2014.
About Parsemus Foundation
Parsemus Foundation works to create meaningful improvements in human and animal health and welfare by advancing innovative and neglected medical research, with an emphasis on making sure advances change treatment practice rather than disappearing into the scientific literature. Many of the studies the foundation supports involve low-cost approaches that are not under patent. Focus areas include reproductive health, animal reproductive health, and COVID-19 prevention and treatment. More information on the Parsemus Foundation and its work can be found at http://www.
Media Contact
Linda Brent
[email protected]
Related Journal Article
http://dx.