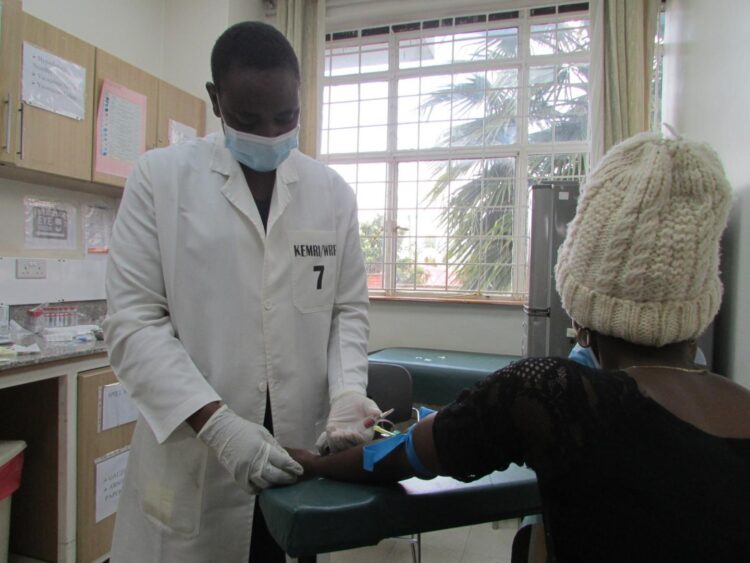
The first vaccine has been administered in a comparative adjuvant trial of DNA prime/protein boost HIV vaccine regimens in Kericho, Kenya
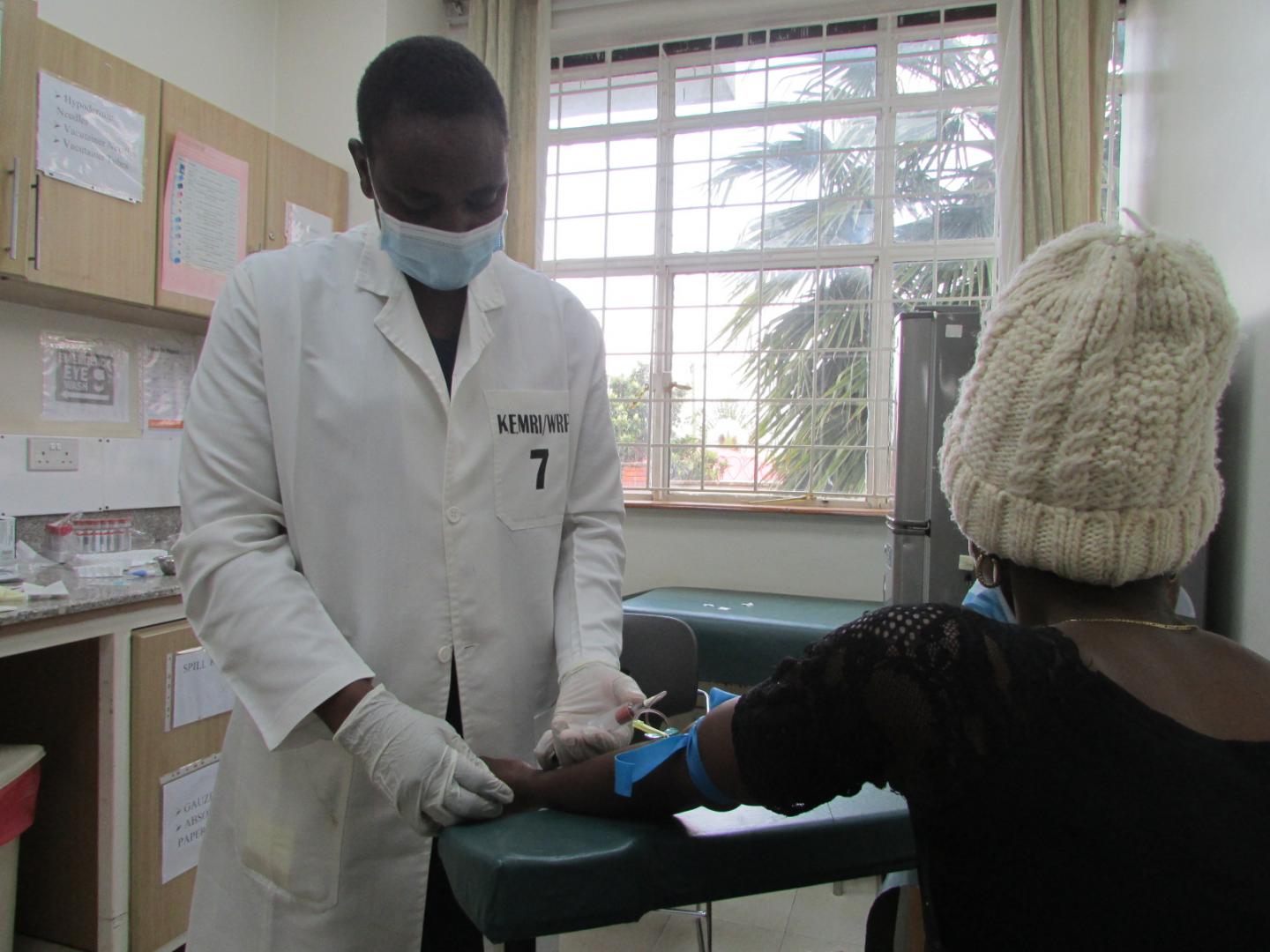
Credit: U.S. Military HIV Research Program
SILVER SPRING, Md. – A new Phase 1 vaccine study began today that will evaluate experimental prime/boost HIV-1 vaccine regimens formulated with combinations of different adjuvants, including one from the Army Liposome Formulation (ALF) family of adjuvants developed by scientists with the U.S. Military HIV Research Program (MHRP) at the Walter Reed Army Institute of Research.
An adjuvant is a component of a vaccine that can help make it more effective by improving the immune response or causing the immune response to last longer. The goal of the new trial, RV460, is to evaluate whether an adjuvant can improve immune response to the antigens in the candidate vaccines. It will also help characterize the differences between the adjuvants and the role of adjuvants in priming versus boosting.
“By testing a DNA vaccine in combination with protein boosts and adjuvants, we hope to inform a more promising vaccine strategy that will elicit stronger, more durable immune responses,” said MHRP Director Col. Julie Ake.
The study is led by MHRP scientists in partnership with the Kenya Medical Research Institute/Walter Reed Project Clinical Research Center in Kericho, where the study will take place. The regimens being compared comprise an HIV-1 env-C plasmid DNA vaccine followed by a gp145 protein vaccine boost, and three different adjuvant products will be compared.
One of the adjuvants is ALFA, which was developed by U.S. Army scientists in MHRP’s Laboratory of Adjuvant and Antigen Research. In a 2020 preclinical study conducted by MHRP, an HIV vaccine formulated with AFLA elicited stronger immune responses than a vaccine formulated with a more commonly used alum adjuvant.
“Adjuvants work by recruiting immune cells into the site of immunization, which take up the HIV antigen and carry it to lymph nodes to make strong immune responses,” explained Dr. Gary Matyas, chief of MHRP’s Adjuvants and Formulations lab section. “We hope that the types of adjuvants used in RV460 will increase the immunity generated by DNA vaccines.”
A total of 126 healthy adult participants will enroll in the study. Participants will be randomly assigned into one of seven groups, and each individual will receive prime injections at week 0, 4, and 12, and boost injections at week 20, 32 and 56.
The comparative study may have impact beyond the HIV field. According to the study’s principal investigator, Dr. Josphat Kosgei, “this adjuvant study should be generalizable, and might inform vaccines for other pathogens.”
###
About the trial:
RV460 is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS (DAIDS). Funding is provided by a cooperative agreement between Congressionally Directed Medical Research Programs (CDMRP) – United States Army Medical Research Acquisition Activity (USAMRAA), U.S. Military HIV Research Program and Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) Cooperative Agreement (CA# W81XWH-18-2-0040). The trial is registered on clinicaltrials.gov: https:/
About MHRP
The U.S. Military HIV Research Program (MHRP), part of the Walter Reed Army Institute of Research, is at the forefront of the battle against HIV/AIDS to protect U.S. troops from infection and reduce the global impact of the disease. MHRP has been recognized as a leader in HIV diagnostics, epidemiology and vaccine research for over three decades and, more recently, has built an international reputation for pioneering discoveries in acute infection and remission. Its research is supported by a cooperative agreement (W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF), and the U.S. Department of Defense (DOD). The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or HJF.
Media Contact
Lisa Reilly
[email protected]
Original Source
https:/