Credit: WANG Jiawang
Recently, research group, led by Prof. FU Yao and associate research fellow LU Xi From Hefei National Laboratory for Physical Sciences at the Microscale and School of Chemistry and Materials Science of the University of Science and Technology of China (USTC), has made significant achievements in the field of synthesis of chiral amines. They developed a mild and general nickel-catalysed asymmetric reductive hydroalkylation and realized the modular synthesis of chiral aliphatic amines.
Results were published in Nature Communications on Feb. 26, 2021.
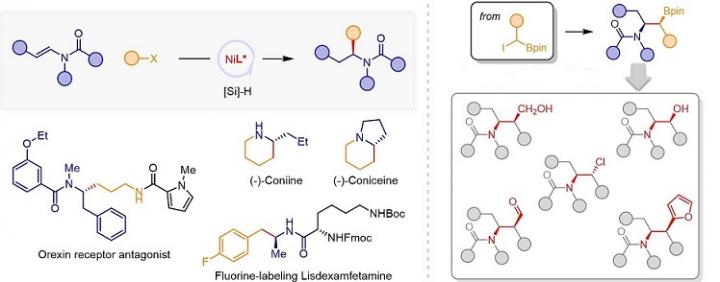
Chiral amines are important chiral auxiliaries and key synthetic intermediates of pharmaceuticals and natural products. It is an important direction to develop efficient and convenient synthesis of chiral amines in organic synthetic chemistry. However, conventional catalytic synthesis methods, such as imine and enamine hydrogenation, imine alkylation, olefin hydroamination and others, have limited substrate structure in the synthesis of dialkyl substituted chiral aliphatic amines.
The research group proposed the concept of olefin reductive coupling and developed a cheap nickel catalytic system to realize carbon carbon coupling under mild conditions by replacing equivalent metal reagents with olefins.
In this work, they combined saturated carbon couple, and successfully realized the asymmetric hydroalkylation of enamides catalyzed by Ni MH. In addition, the asymmetric hydroalkylation of enamide with α-borate halide can be realized to prepare β-aminoborate with two chiral centers. Tandem conversion of organic boron compounds will contribute to the development of amine chiral molecules with complex structures.
This work is a complement and breakthrough for the structural limitations of conventional chiral amine synthesis strategies.
###
Media Contact
Jane FAN Qiong
[email protected]
Related Journal Article
http://dx.




