Credit: Carnegie Mellon University
Researchers at Carnegie Mellon University have used nuclear resonance vibrational spectroscopy to probe the hydrogen bonds that modulate the chemical reactivity of enzymes, catalysts and biomimetic complexes. The technique could lead to the development of better catalysts for use in a wide range of fields. The findings were published as a “Very Important Paper” in the Dec. 3 issue of Angewandte Chemie and featured on the journal’s back cover.
Hydrogen bonds are among the most fundamental interactions found in biology and chemistry. They are responsible for many of the chemically important properties of water, for the stabilizing the structures of proteins and nucleic acids, including those found in DNA and RNA, and contribute to the structure of natural and synthetic polymers.
Research has shown that hydrogen bonds play an important role in tuning the reactivity of the metal centers of metalloenzymes and metal containing catalysts. However, little research has been done to experimentally demonstrate how systematic changes to hydrogen bonds within the secondary coordination sphere — molecules found in the vicinity of metal centers that do not have direct bonding interactions with the center — influence catalytic activity.
In catalysis, enzymes or synthetic catalysts spur on a chain of chemical reactions, which produce a number of intermediate structures or species. Understanding those structures and their chemical properties is key to understanding the entire reaction.
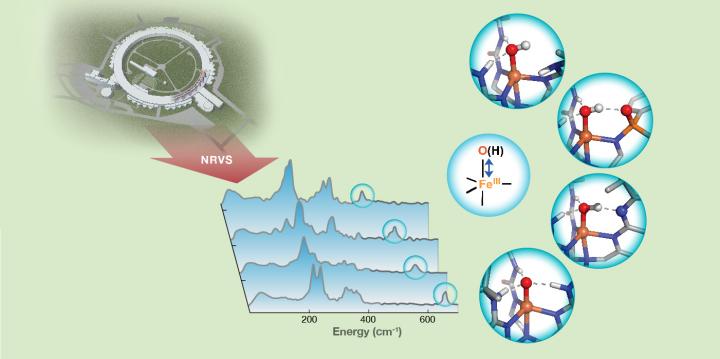
“Thoroughly understanding the chemical reactivity of the reactive intermediate is a key step to determining how to design highly efficient and selective catalysts for C-H functionalization,” said Yisong Guo, assistant professor of chemistry at Carnegie Mellon and the study’s lead author. “In the case of dioxygen-activating enzymes, the key intermediates of catalysis are iron-oxo (Fe-O) and iron-hydroxo (Fe-OH) species, which are involved in important biological processes, such as DNA biosynthesis, DNA and RNA repair, post-translational modification of proteins, biosynthesis of antibiotics and degradation of toxic compounds.”
Guo and colleagues used 57Fe nuclear resonance vibrational spectroscopy (NRVS), a newly developed synchrotron radiation-based technique, to detect the vibrational frequency of Fe-O and Fe-OH units of synthetic complexes that interact with the secondary coordination sphere through hydrogen bonds. Changes in the frequencies revealed valuable information about the bond strengths of these units and further provided a qualitative measure of hydrogen bond strength.
“This showed that NRVS is a sensitive technique to pick up very small changes in hydrogen bond strength, down to the changes of a single hydrogen bond. This provides us with a new method to connect changes in bond strength of Fe-O and Fe-OH units to their chemical reactivity,” said Guo.
Guo says that this study is a proof-of-concept for using NRVS to probe hydrogen bonds. He plans to continue using this method to study more iron-oxo and iron-hydroxo species in both synthetic complexes and enzymes to build up the amount of available data to correlate chemical reactivity of these species with the changes of hydrogen bond interactions, with the hope that that information could be used to develop more efficient and effective catalysts.
###
All of the experimental data in this study was recorded at the Advanced Photon Source at Argonne National Laboratory, which is supported by the Department of Energy. Computation was completed using the Extreme Science and Engineering Discovery Environment (XSEDE) and Bridges System at the Pittsburgh Supercomputing Center, which is funded by the National Science Foundation (NSF). The research was funded by the National Institutes of Health (GM050781, GM077387) and NSF (1654060).
Study authors include Guo, Andrew C. Weitz, Emile M. Bominaar and Michael P. Hendrich from the Carnegie Mellon University and Andrew S. Borovik, Ethan A. Hill and Victoria F. Oswald from the University of California, Irvine.
Media Contact
Jocelyn Duffy
[email protected]
412-268-9982
Original Source
https:/
Related Journal Article
http://dx.
News source: https://scienmag.com/




