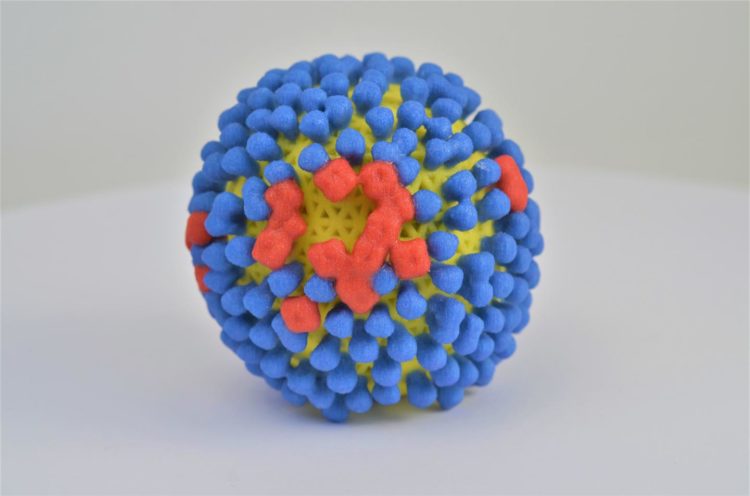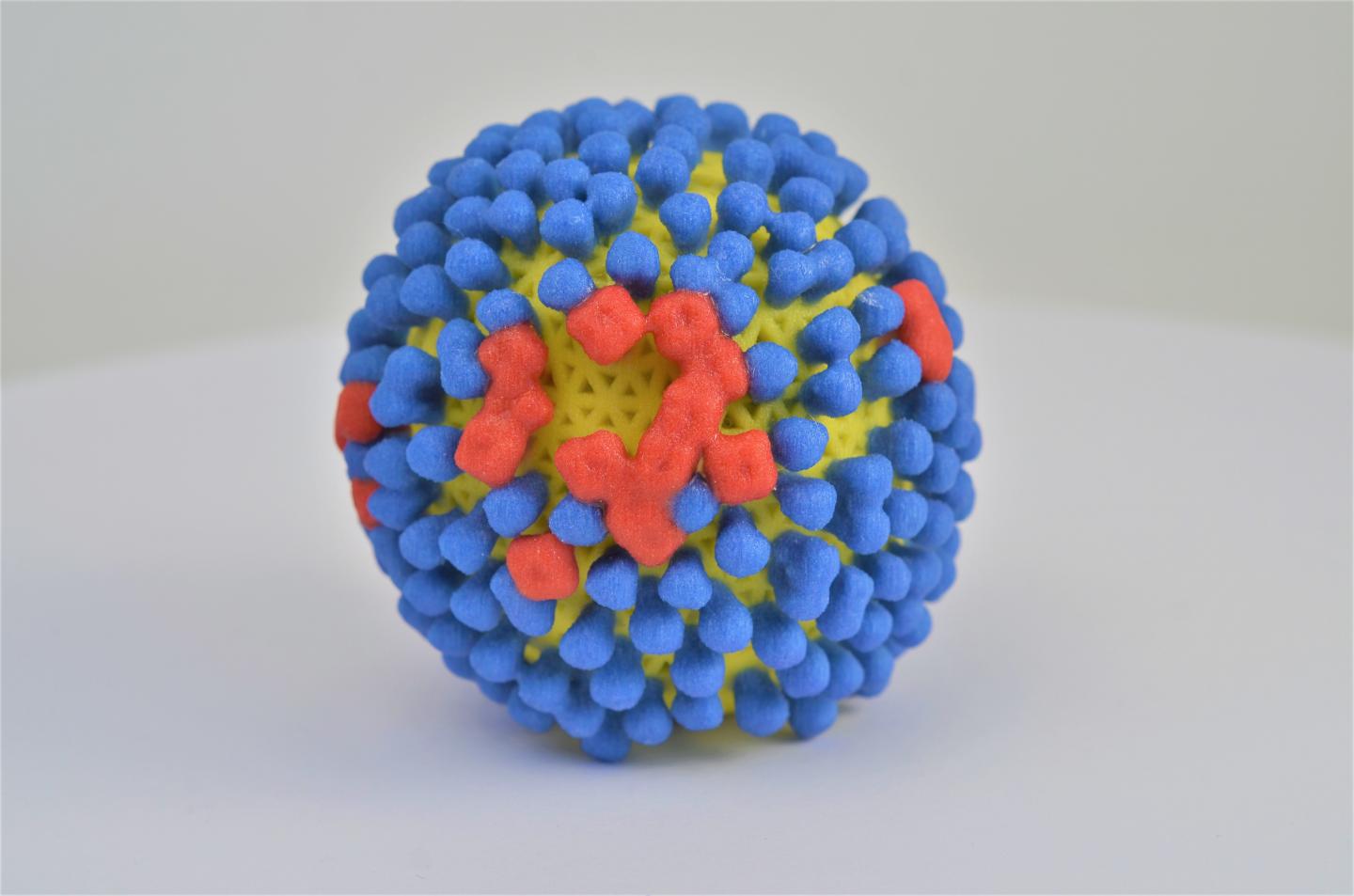
Credit: NIAID
WHAT:
A newly identified set of three antibodies could lead to better treatments and vaccines against influenza, according to a paper published this week in Science. Researchers supported by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, isolated the antibodies from a person sick with the flu five days after the onset of symptoms. They found that the antibodies, which bind to neuraminidase (NA) proteins on the surface of influenza viruses, provided broad protection against several different strains of influenza when tested both in vitro and in mice.
Most influenza vaccines are designed to stimulate an immune response against another protein found on the surface of the influenza virus called hemagglutinin (HA). However, HA proteins change frequently as the virus evolves. As a result, people must receive a new seasonal influenza vaccination every year to be protected against currently circulating influenza viruses. NA proteins change more slowly than HA proteins and thus could be a good target for an influenza vaccine that provides long-term protection.
In the new study, the researchers took antibody-producing cells from the blood of a volunteer sick with H3N2 influenza and screened for monoclonal antibodies (mAbs). Monoclonal antibodies are antibodies which are designed to bind to a single target. The mAbs the researchers found were tested in the laboratory for their ability to bind to different kinds of influenza proteins. Of 45 mAbs tested, three bound to NA proteins of an H3N2 influenza virus strain. Upon further testing, these three mAbs also bound to NA proteins from multiple other types of influenza viruses.
To see whether these three mAbs could help prevent the influenza virus from infecting mammalian cells, the researchers treated mice with the mAbs and then infected them with different types of influenza viruses. The mAbs inhibited many kinds of NA proteins from different types of influenza viruses, and protected most of the mice from severe influenza infections. Mice given lethal doses of H3N2 influenza virus survived when treated with low doses of the three antibodies.
If additional testing supports these early results, the researchers suggest that these potent mAbs could become the basis for a new antiviral treatment. Additionally, the antibodies could inform development of new influenza vaccines designed to induce similar antibodies that could provide broader and longer-lasting immunity than current HA-based influenza
###
This research was supported in part by NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contracts (HHSN272201400008C and HHSN272201400006C), and several NIAID grants: R01 AI117287, R21 AI139813, U01 AI141990 and R56 AI117675.
ARTICLE:
Stadlbauer et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science. DOI: 10.1126/science.aay0678 (2019).
WHO:
NIAID Director Anthony S. Fauci, M.D., is available for comment.
CONTACT:
To schedule interviews, please contact Elizabeth Deatrick, (301) 402-1663, [email protected].
NIAID conducts and supports research–at NIH, throughout the United States, and worldwide–to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
Media Contact
Elizabeth Deatrick
[email protected]
301-402-1663
Original Source
https:/
Related Journal Article
http://dx.