Rice scientists lead effort to improve manufacture of valuable 2D material
Credit: Zhuhua Zhang/Rice University/Nanjing University of Aeronautics and Astronautics
HOUSTON – (Sept. 27, 2019) – Borophene has a nearly perfect partner in a form of silver that could help the trendy two-dimensional material grow to unheard-of lengths.
A well-ordered lattice of silver atoms makes it possible to speed the growth of pristine borophene, the atom-thick allotrope of boron that so far can only form via synthesis by molecular-beam epitaxy (MBE).
By using a silver substrate and through careful manipulation of temperature and deposition rate, scientists have discovered they can grow elongated hexagon-shaped flakes of borophene. They suggested the use of a proper metal substrate could facilitate the growth of ultrathin, narrow borophene ribbons.
New work published in Science Advances by researchers at Rice and Northwestern universities, Nanjing University of Aeronautics and Astronautics and Argonne National Laboratory will help streamline the manufacture of the conductive material, which shows potential for use in wearable and transparent electronics, plasmonic sensors and energy storage.
That potential has fueled efforts to make it easier to grow, led by Rice materials scientist Boris Yakobson, a theorist who predicted that borophene could be synthesized. He and collaborators Mark Hersam at Northwestern and lead author Zhuhua Zhang, a Rice alumnus and now a professor at Nanjing, have now demonstrated through theory and experimentation that large-scale, high-quality samples of borophene are not only possible but also allow qualitative understanding of their growth patterns.
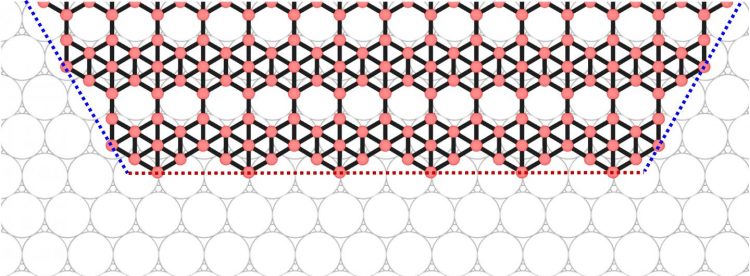
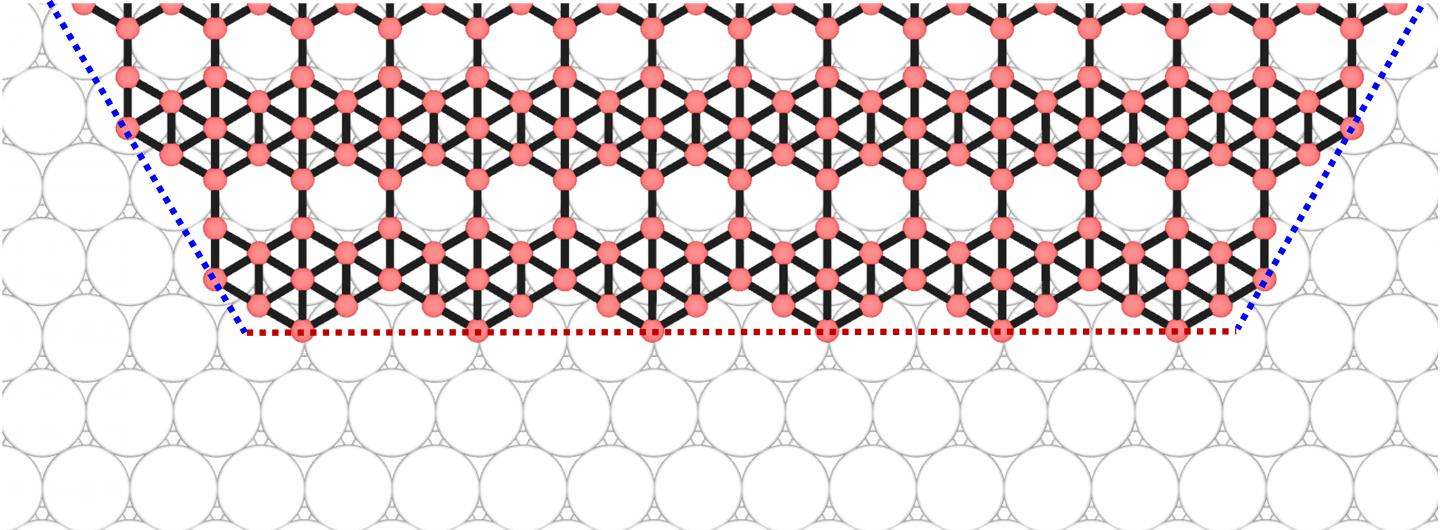
Unlike the repeating atomic lattices found in graphene and hexagonal boron nitride, borophene incorporates a regular, woven-in array of “vacancies,” missing atoms that leave hexagonal holes among the triangles. This not only affects the material’s electronic properties but also influences how new atoms join the flake as it is being formed.
The Yakobson lab’s calculations showed the edge energies — atoms that are less stable along the edges of 2D materials than those in the interior — are significantly lower than those in graphene and boron nitride and that the conditions can be manipulated to tune the edges for optimum growth of ribbons.
Initial calculations showed borophene in equilibrium should form as a rectangle, but experiments proved otherwise.
The confounding factor was in the flake’s edges that, forced by the vacancies, appear in variations of zigzag and armchair configurations. Atoms settle one by one into the “kinks” that appear along the edges, but as armchairs are more energetically stable and present a higher barrier to the atoms, they prefer to join the zigzags. Rather than extending the flakes in all directions, the atoms are selective about where they settle and elongate the structure instead.
“On the atomic scale, edges don’t act as though you cut the lattice with a pair of scissors,” Yakobson said. “The dangling bonds you create reconnect with their neighbors, and the edge atoms adapt slightly different, reconstructed configurations.
“So the origin of the shapes must not lie in equilibrium,” he said. “They are caused by the kinetics of growth, how fast or slow the side edges advance. Opportunely, we had developed a theoretical framework for graphene, a nanoreactor model that works for other 2D materials, including boron.”
Controlling the flow of atoms as well as temperature gives the researchers a simpler way to control borophene synthesis.
“Silver (111) provides a landing for boron atoms, which then diffuse along the surface to find the edges of a growing borophene flake,” Zhang said. “Upon arrival, the boron atoms are lifted onto the edges by silver, but how difficult such a lift is depends on the edge’s orientation. As a result, a pair of opposite zigzag edges grow very slowly while all other edges grow very fast, manifested as an elongation of the boron flake.”
The researchers said the ability to grow needlelike ribbons of borophene gives them the potential to serve as atom-width conductive wires for nanoelectronics devices.
“Graphene-based electronics that have been conceived so far mostly rely on ribbonlike building blocks,” Yakobson said. “Metallic boron ribbons with high conductivity will be a natural match as interconnects in circuitry.”
###
Co-authors of the paper are Xiaolong Liu of Northwestern, Nathan Guisinger of Argonne’s Center for Nanoscale Materials, Andrew Mannix of Argonne and Northwestern, and Zhili Hu of Nanjing and Rice. Yakobson is the Karl F. Hasselmann Professor of Materials Science and NanoEngineering and a professor of chemistry at Rice. Hersam is the Walter P. Murphy Professor of Materials Science and Engineering at Northwestern.
The National Natural Science Foundation of China, the State Key Laboratory of Mechanics and Control of Mechanical Structures, the Department of Energy, the Office of Naval Research and the National Science Foundation supported the research.
Read the abstract at https:/
This news release can be found online at https:/
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Every atom counts in graphene formation: http://news.
Gold soaks up boron, spits out borophene: http://news.
2D borophene gets a closer look: http://news.
In borophene, boundaries are no barrier: http://news.
Yakobson Research Group: https:/
Hersam Research Group: http://www.
Images for download:
https:/
An illustration shows how edges are connected at the corners of a borophene flake. Materials scientists led by Rice University have predicted that the shape of borophene, the 2D allotrope of boron, can be controlled. (Credit: Zhuhua Zhang/Rice University/Nanjing University of Aeronautics and Astronautics)
https:/
Models by materials scientists at Rice University and their colleagues suggest a method to control the growth of valuable two-dimensional borophene. The model predicts a variety of possible shapes of the 2D boron flakes with different aspect ratios, including thin ribbons. (Credit: Zhuhua Zhang/Rice University/Nanjing University of Aeronautics and Astronautics)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Media Contact
Mike Williams
[email protected]
Original Source
https:/
Related Journal Article
http://dx.