Credit: Copyright © 2020 Yosuke Kageshima, Shinshu University
Water splitting research for solar hydrogen production has focused on physical processes inside the semiconductor, such as light absorption, charge separation, and chemical processes on the surface that are highly complex and rely on the development of new materials. However, processes inside the solution had yet to be thoroughly explored.
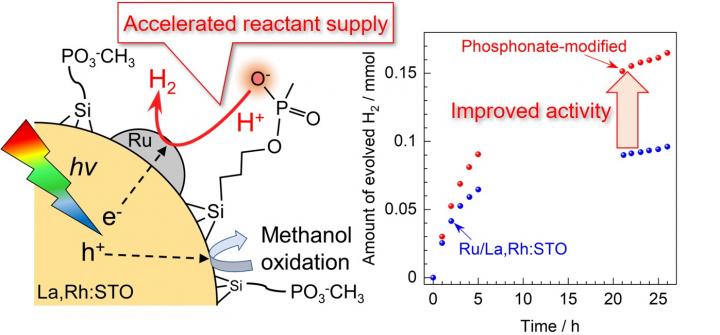
One recent approach to improve photocatalytic hydrogen production was proposed by loading phosphonate groups on the surface of the visible-light-responsive photocatalyst lanthanum and rhodium-doped strontium titanate (La,Rh:STO) with a silane coupling agent. The phosphonate functional group functions as a mediator of proton supply (i.e., promotes the supply of reactants) and improves hydrogen production activity.
There have been examples of research using unbuffered electrolyte or phosphate buffer solution as reaction solution in photocatalytic water splitting reaction. However, the former is mostly an electrostatic interaction between photocatalytic material and ions in the solution, while the latter focuses on the function of the phosphate anion as a proton mediator. These are equivalent to the design of bulk electrolyte.
The concept proposed in this study is neither development of the material itself nor design of the bulk electrolyte, but design of the electrolyte-photocatalyst interface: a new concept that enables control of physicochemical phenomena in solution, especially in the vicinity of the catalyst surface via functional groups immobilized on the photocatalyst powder surface. The phosphonate functional groups immobilized on the surface of the powdered photocatalyst effectively supply protons to the active site. The phosphonate functional groups immobilized on the surface of the powdered photocatalyst contribute to the enhancement of hydrogen production activity by functioning as mediators to effectively supply protons to the active site.
To the delight of lead researcher Yosuke Kageshima, when the bulk phosphate buffer solution was used as the reaction solution, the hydrogen production activity of La,Rh:STO was greatly reduced. Although it is common to focus on the function of phosphate anions as proton mediators, the approach of immobilizing functional groups near the solid-liquid interface can be a broad-ranging methodology that is effective regardless of the materials used.
Further improvement of activity by increasing the concentration of phosphonate functional groups immobilized on the surface will be explored in further studies. Detailed quantitative evaluation of the diffusion process of phosphonate functional groups are needed as well as expansion for use in overall water decomposition reaction. Assistant Professor Kageshima hopes to construct a stand-alone artificial photosynthesis device that contributes to the realization of a low-carbon society through the practical application of solar hydrogen production, which converts solar energy into chemical energy that is advantageous for storage and transportation.
###
Shinshu University has recently been involved in a string of breakthroughs in photocatalytic hydrogen production through water splitting with solar energy. Please read Solar hydrogen production for more information.
This study was supported by Japan Society for the Promotion of Science.
The original paper in Angewandete Chemie:
Boosted Hydrogen Evolution Kinetics Over Particulate Lanthanum and Rhodium?Doped Strontium Titanate Photocatalysts Modified with Phosphonate Groups
Media Contact
Hitomi Thompson
[email protected]
Related Journal Article
http://dx.




