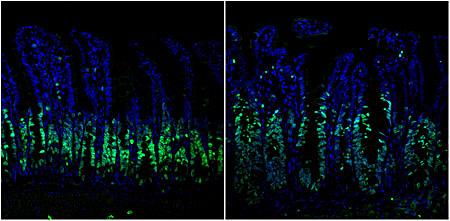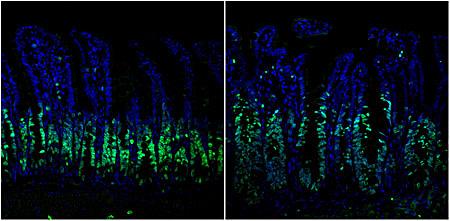
While investigating a potential therapeutic target for the ERK1 and 2 pathway, a widely expressed signaling molecule known to drive cancer growth in one third of patients with colorectal cancer, University of California San Diego School of Medicine researchers found that an alternative pathway immediately emerges when ERK1/2 is halted, thus allowing tumor cell proliferation to continue.
“Since we were genetically deleting the ERK1/2 pathway, we expected to see less cell proliferation,” said Petrus R. de Jong, MD, PhD, a co-first author on the paper. “Instead, the opposite occurred. There was more cell growth and loss of organization within the cells.”
In a paper published online May 17, 2016 in the journal Nature Communications, de Jong and co-first author Koji Taniguchi, MD, PhD, and colleagues, report that treating both ERK1/2 and the compensatory pathway, ERK5, concurrently with a combination of drug inhibitors halted colorectal cancer growth more effectively in both mouse models and human colorectal cancer cell lines.
The ERK pathway plays a critical role in embryonic development and tissue repair because it instructs cells to multiply and start dividing, but when over activated cancer growth occurs.
“Therapies aimed at targeting ERK1/2 likely fail because this mechanism is allowing proliferation through a different pathway,” said Eyal Raz, MD, senior author and UC San Diego School of Medicine professor of medicine. “Previously, ERK5 didn’t seem important in colorectal cancer. This is an underappreciated escape pathway for tumor cells. Hence, the combination of ERK1/2 and ERK5 inhibitors may lead to more effective treatments for colorectal cancer patients.”
There are 1.2 million people living with this disease in the United States, making it the third most common cancer among men and women. In 2016, an estimated 134,490 new cases are expected to be diagnosed.
“If you block one pathway, cancer cells usually mutate and find another pathway that ultimately allows for a recurrence of cancer growth,” said Taniguchi. “Usually, mutations occur over weeks or months. But other times, as in this case, the tumor does not need to develop mutations to find an escape route from targeted therapy. When you find the compensatory pathway and block both, there is no more escape.”
Researchers suggested that other classes of inhibitors be tested in combination with ERK5 inhibitors in human colorectal cancer cells in preclinical mouse models before any patient trial can begin.
###
Additional study co-authors include: Alexandra Harris, Samuel Bertin, Naoki Takahashi, Jen Duong, Maripat Corr, Michael Karin, UC San Diego; and Alejandro Campos and Garth Powis, Sanford Burnham Prebys Medical Discovery Institute.
Media Contact
Yadira Galindo
[email protected]
619-543-6163
@UCSanDiego
http://www.ucsd.edu
The post Blocking known cancer driver unexpectedly reveals a new tumor-promoting pathway appeared first on Scienmag.