Credit: Kirill Kovalev et al./Science Advances
A team of biophysicists from Russia, Germany, and France, featuring researchers from the Moscow Institute of Physics and Technology, has discovered and studied the structure of the KR2 rhodopsin under physiological conditions. This pioneering work breaks ground for a future breakthrough in optogenetics, a highly relevant area of biomedicine with applications in neurological disease treatment and more. The fundamental discovery will lead to a new instrument for efficient therapy of depression, anxiety disorders, epilepsy, and Parkinson’s disease. The paper reporting the study, in which MIPT biophysicists played a leading part, was published in Science Advances, a highly regarded journal of the American Association for the Advancement of Science.
Optogenetics is an entirely new area of biophysics and biomedicine, which investigates techniques for controlling the nerve and muscle cells in a living organism via light signals. Not long ago, the leading research journal Science hailed optogenetics as the “breakthrough of the decade.” Optogenetic methods already enable a partial recovery of lost eyesight, hearing, and muscle control impaired by a neurological disease. Importantly, these techniques allow researchers to study neural networks in detail. This refers not to computer networks but to those housed in the human brain and responsible for our emotions, decision-making, and other fundamental processes.
Several years ago, researchers discovered a new type of ion transporter — the KR2 rhodopsin — in the cell membrane of the marine bacterium Krokinobacter eikastus. The newly found protein is sensitive to light, making it useful for optogenetics. Driven by light, such proteins can facilitate the translocation of charged particles such as ions across the cell membrane. By introducing such transporters into the cell, researchers can then use light pulses to manipulate the potential of the neuron cell membrane, controlling its activity. KR2 was shown to selectively transport a particular kind of particles — sodium ions — outside the cell. Rather than allow the passage of these ions in both directions, the protein performs active transport, serving as a “pump.” Mutant forms of KR2 also showed potassium-pumping activity. By implanting these pumps into the cell membrane, the whole scope of neuron activity could theoretically be controlled.
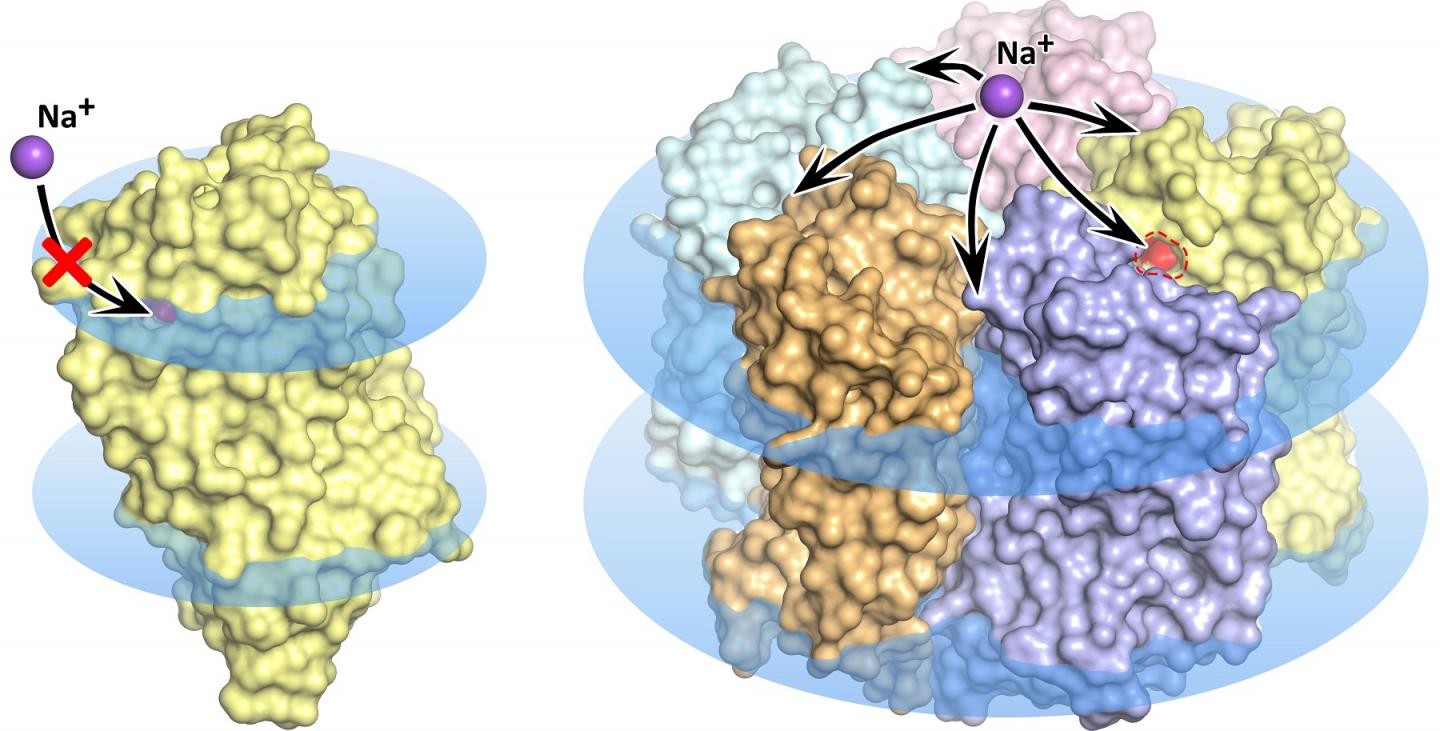
The wave of research that followed the discovery of the new molecular pump faced some pretty mysterious properties of the rhodopsin. Several research groups discovered and described a total of five different structures of the promising protein. Notably, in some of these structures five KR2 molecules form a stable pentamer, while in others only the protein monomer is present (figure 1).
“So the dramatic question was: Which of these structures should be considered the right one?” said MIPT doctoral student Kirill Kovalev, a lead author of the study. “In fact, the structures turned out to be pretty similar, but the devil’s in the details, which determine the protein’s possible applications in science and the clinical practice.”
Led by MIPT biophysicists, the team found what gives rise to the confusing variety of protein structures. It turned out that the research groups studying KR2 had crystallized the protein at different conditions. The unique protein is originally produced by an ocean bacterium native to a very special environment. It lives in water with a specific salinity, acidity, and hydrogen ion concentration (pH). These conditions are a prerequisite for the protein to do what researchers expect it to do — that is, pump sodium ions, while also forming pentamers in the cell membrane. The protein’s numerous “false” structures turned out to either be crystallization artifacts or only correspond to the conditions that virtually disable the sodium-pumping activity of KR2, which makes it highly attractive for the global optogenetics community.
“For the first time, we have simulated the physiological conditions for KR2 existence and functioning. As a result, we obtained the ‘correct’ structure of the new protein, which corresponds to its native state. We showed that the functional unit of the protein is a pentamer,” explained Valentin Gordeliyfrom the Institute of Structural Biology in Grenoble. “On top of that, we found an explanation for the contradictions between previous structural studies of the protein.”
The KR2 rhodopsin is revolutionary for optogenetics, and knowing its correct structure under physiological conditions is fundamental both for understanding the mechanisms behind its functioning and for exploring the nervous system by modeling new optogenetic tools and applying them in the medical practice.
###
The study reported in this story featured researchers from the Moscow Institute of Physics and Technology, the Institute of Structural Biology of Grenoble University, the European Synchrotron Radiation Facility, Research Center Juelich, the University of Aachen, and Max Planck Institute of Biophysics.
Media Contact
Ilyana Zolotareva
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




