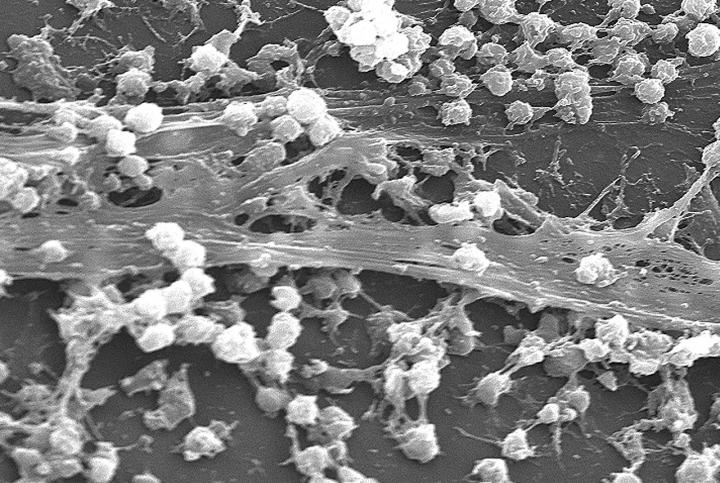
Biofilms, not visible by the naked eye, go undetected by health professionals damaging healing tissue and causing delays in wound healing by reducing the susceptibility of microorganisms to antibiotic, antimicrobial and host immune treatments
Credit: University of Huddersfield
WOUND care experts at the University of Huddersfield have joined forces with industry to pool their expertise for a research project that aims to not only significantly improve quality of life for those suffering with chronic wound infections but also has the potential to save the global healthcare industry millions of pounds.
The University’s Institute of Skin Integrity and Infection Prevention (ISIaIP) has teamed up with Perfectus Biomed, one of the UK’s leading testing laboratories, to investigate ways for wound care clinicians to accurately identify and manage the presence of biofilms – known as microscopic communities of bacteria – within chronic wounds or infection sites.
Biofilms are generally composed of bacteria, fungi and other micro-organisms that attach to and grow on surfaces. Plaque which forms on teeth and causes tooth decay is one type of bacterial biofilm. Early detection is essential because if left untreated biofilms can significantly impact a wound’s ability to heal by contributing to bacterial infection, inflammation and can cause delayed wound healing.
The team of researchers who will be working on the project consists of the Director of the University’s award-winning ISIaIP, Professor Karen Ousey, the Head of Pharmacy Professor Barbara Conway and Dr Leanne Atkin, a practising Vascular Nurse Consultant at Pinderfields Hospital and part-time University lecturer.
They will be working alongside Dr Samantha Westgate and her team at Perfectus Biomed for the collaborative research project entitled Development of molecular support to detect biofilm causing pathogens within chronic infections.
Although over 60% of chronic wounds contain a biofilm, signs of early stage biofilm infection are not visible to the human eye. As a result, many healthcare professionals are not able to identify their presence in time for the patients to receive the most effective treatments.
“Currently swab samples are taken from patients in order to determine the presence of infection, however the biofilm forming nature of the organisms is not assessed,” said Professor Ousey. “This leaves clinicians unable to accurately confirm the early presence of a biofilm infection within a chronic wound.”
Biofilms delay healing process
When a biofilm goes undetected and is left to mature within a chronic wound, the wound can present itself in ways that suggest the presence of a biofilm. However, argues Professor Ousey, there is ongoing disagreement and confusion over whether it is possible to visualise a biofilm with the naked eye, since it is a microscopic entity.
Studies have shown when a biofilm is present it works to reduce the susceptibility of microorganisms to antibiotic, antimicrobial and host immune treatments. This is achieved via a physical barrier that protects the residing organisms from the treatment and through a reduced microbial metabolism it hinders the efficacy of the treatment coming into contact with the biofilm.
“Ultimately, this creates a situation where the body is ineffectively fighting the organisms involved in the biofilm whilst also damaging healing tissue and causes a delay in the wound healing,” said Professor Ousey.
“This is why it is imperative for clinicians to have the ability to diagnose them early,” she added.
This research project is funded by the National Biofilms Innovation Centre which exists to create a fusion of world-class interdisciplinary research and industry partnerships to deliver breakthrough science and technologies to control and exploit biofilms.
###
Media Contact
Karen Ousey
[email protected]
Original Source
https:/




