A review of advances in biochip technology shows it’s playing a fundamental role promoting the use of next-generation DNA sequencing to solve real-world problems in biology and medicine.
Credit: Amos Chungwon Lee
WASHINGTON, D.C., June 25, 2019 — Biochips are essentially tiny laboratories designed to function inside living organisms, and they are driving next-generation DNA sequencing technologies. This powerful combination is capable of solving unique and important biological problems, such as single-cell, rare-cell or rare-molecule analysis, which next-generation sequencing can’t do on its own.
Now that the scaling and throughput power of biochip technologies has emerged, the next trend in biochips will involve being capable of providing applications across a wide spectrum — from identifying rare bacterium to population-based clinical studies.
In APL Bioengineering, from AIP Publishing, a group of researchers from Seoul National University explore the role advancements in biochip technology are playing in driving groundbreaking scientific discoveries and breakthroughs in medicine via next-generation sequencing, aka high-throughput sequencing.
“There have been many experimental successes and failures within the biochip field, and one of the most important things we point out in our review is that these were not done in vain,” said Amos Chungwon Lee, lead author and a graduate student in Sunghoon Kwon’s Biophotonics and Nano Engineering Laboratory. “These technologies are now moving forward and being applied in real-world settings.”
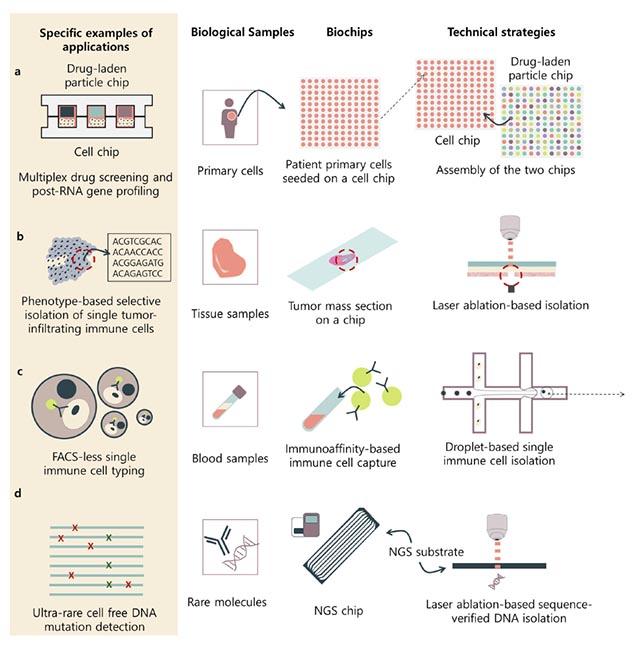
One example of real-world use is biochips that isolate single cells from a heterogeneous mix of a complex biological mass — they are enabling preprocessing for massively parallel $1 single-cell analysis.
“When these biochips meet next-generation sequencing, the net value of both technologies will increase exponentially,” Lee said. “If biochips that allow single-cell analysis prevailed for the last decade, there are many more biochips with different functions that will now innovate the bio field — including drug-screening biochips.”
In their paper, the researchers provide a review of current state-of-the-art biochip technologies, separated into two main categories: microfluidic- and micromanipulation-based methods.
“Microfluidic-based methods use micrometer-sized fluidic channels to compartmentalize biological targets to analyze each and every target, while micromanipulation-based methods use optical devices to pick out the biological target of interest,” explained Lee. “The biggest differences between these methods are the throughput and depth. Microfluidic-based methods have higher throughput, but the micromanipulation-based methods can provide deeper data for the biological targets.”
In the future, biochips will become widely available to the public.
“Like smartphones, biochips will be used by people on a daily basis to check their health or nutritional status,” said Lee. “In the same way that smartphones have shifted the paradigm for information flow, smart biochips will play a key role in revolutionizing the system for the acquisition and interpretation of Mother Nature’s information flow.”
###
The article, “Divide and conquer: A perspective on biochips for single-cell and rare-molecule analysis by next-generation sequencing,” is authored by Amos C. Lee, Yongju Lee, Daewon Lee and Sunghoon Kwon. It appeared in APL Bioengineering on June 25, 2019 (DOI: 10.1063/1. 5095962) and can be accessed at http://aip.
ABOUT THE JOURNAL
APL Bioengineering is devoted to research at the intersection of biology, physics, and engineering. The journal publishes high-impact manuscripts specific to the understanding and advancement of physics and engineering of biological systems. APL Bioengineering is the new home for the bioengineering and biomedical research communities. See https:/
Media Contact
Larry Frum
[email protected]
Related Journal Article
http://dx.




