Infection researchers from the German Primate Center identify starting points for vaccine development and therapy
Credit: Figure: Markus Hoffmann
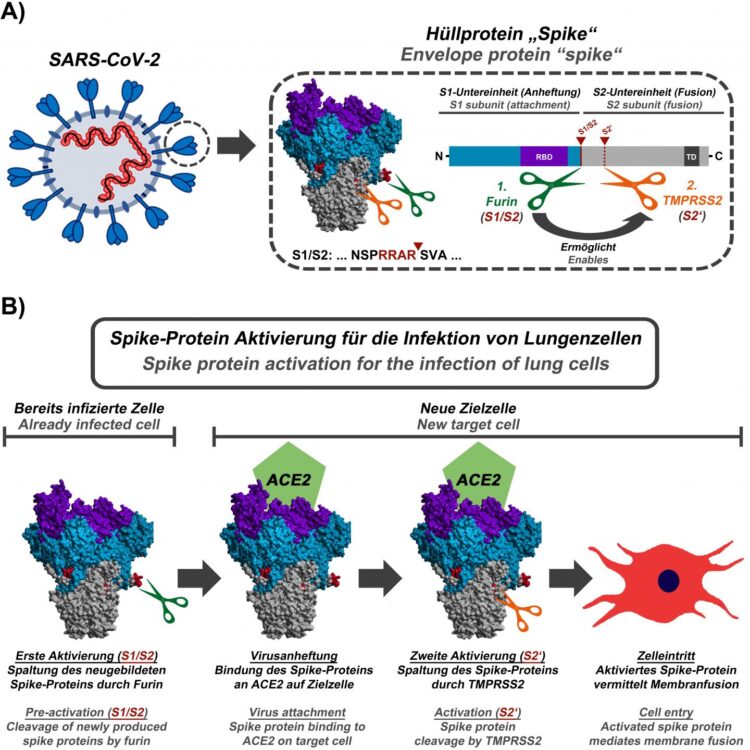
The SARS coronavirus 2 (SARS-CoV-2) infects lung cells and is responsible for the COVID-19 pandemic. The viral spike protein mediates entry of the virus into host cells and harbors an unusual activation sequence. The Infection Biology Unit of the German Primate Center (DPZ) – Leibniz Institute for Primate Research has now shown that this sequence is cleaved by the cellular enzyme furin and that the cleavage is important for the infection of lung cells. These results define new starting points for therapy and vaccine research. In addition, they provide information on how coronaviruses from animals need to change in order to be able to spread in the human population (Molecular Cell).
The new coronavirus SARS-CoV-2 has been transmitted from animals to humans and is spreading worldwide. It causes the new lung disease COVID-19, which has already killed over 200,000 people. The spike protein on the virus surface serves as a key for the virus to enter host cells. It facilitates viral attachment to cells and fuses the viral with a cellular membrane, thereby allowing the virus to deliver its genome into the cell, which is essential for viral replication. For this, activation sequences of the spike protein need to be cleaved by cellular enzymes, called proteases. The spike protein of SARS-CoV-2 carries an activation sequence at the so-called S1/S2 cleavage site, which is similar to those observed in highly pathogenic avian influenza viruses, but which has so far not been found in viruses closely related to SARS-CoV-2. The importance of this sequence for the virus was so far unknown.
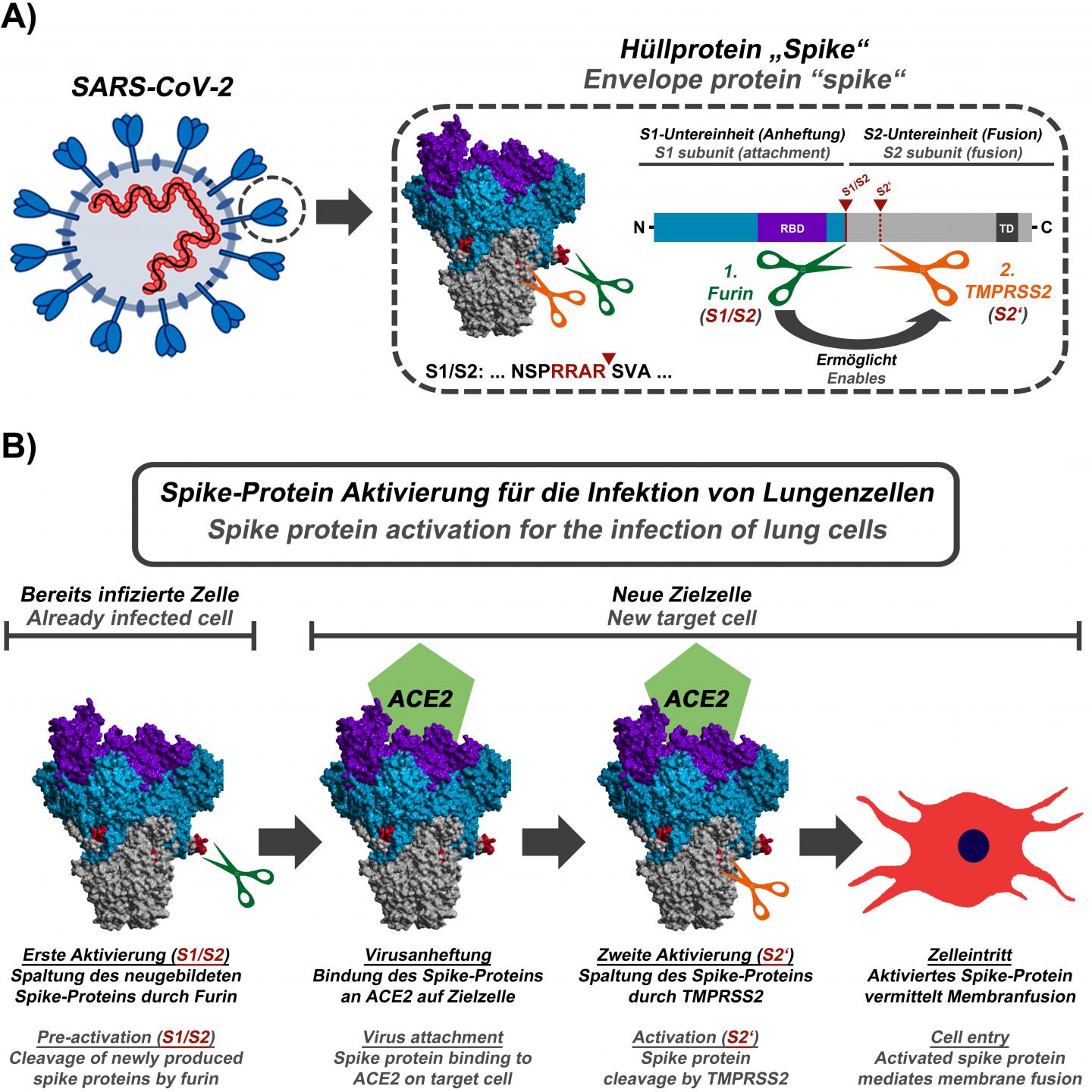
In their current study, the infection biologists of the German Primate Center led by Markus Hoffmann and Stefan Pöhlmann were able to show that the S1/S2 activation sequence of the SARS-CoV-2 spike protein is cleaved by the cellular protease furin and that this cleavage event is essential for the infection of lung cells. It is also important for the fusion of infected cells with non-infected cells, which might allow the virus to spread in the body without leaving the host cell.
“Our results suggest that inhibition of furin should block the spread of SARS-CoV-2 in the lung,” says Stefan Pöhlmann, head of the Infection Biology Unit at DPZ. “Furthermore, our present study and previous work demonstrate that the virus uses a two-step activation mechanism: In infected cells, the spike protein has to be cleaved by the protease furin so that newly formed viruses can then use the protease TMPRSS2 for further cleavage of the spike protein, which is important for the entry into lung cells.”
Development of live attenuated vaccines
For a live attenuated vaccine to trigger a strong immune response, it has to be able to replicate in the body to a limited extent, for example locally at the site of injection. “SARS-CoV-2 variants, in which the activation sequence for furin has been removed, could be used as a basis for the development of such live attenuated vaccines, since the lack of cleavage of the spike protein should greatly limit the spread of the virus in the body. A sufficiently attenuated virus would no longer be able to cause disease, but would still enable the immune system to react to the pathogen and, for example, produce neutralizing antibodies,” says Markus Hoffmann, first author of the study.
Risk assessment
In wildlife, especially bats, a large number of coronaviruses that are closely related to SARS-CoV and SARS-CoV-2 has been discovered over the past 20 years. However, so far an S1/S2 activation sequence that can be cleaved by furin has only been detected in SARS-CoV-2. “Wildlife sampling and the targeted search for coronaviruses with a focus on the S1/S2 activation sequence is necessary to identify those viruses that have the potential to infect and efficiently spread in humans. In addition, in the case of potential future coronavirus outbreaks, we should specifically analyze the S1/S2 cleavage site as it might serve as a marker for human-to-human transmissibility,” says Markus Hoffmann.
###
Contact and notes for editors
Prof. Dr. Stefan Pöhlmann
Tel: +49 551 3851-150
Email: [email protected]
Dr. Markus Hoffmann
Tel: +49 551 3851 338
Email: [email protected]
Dr. Susanne Diederich (Communication)
Tel: +49 551 3851-359
Email: [email protected]
Printable pictures are available here: http://medien.
You will also find the press release on our website (https:/
The German Primate Center GmbH (DPZ) – Leibniz Institute for Primate Research conducts biological and biomedical research on and with primates in the fields of infection research, neuroscience and primate biology. The DPZ also maintains four field stations in the tropics and is a reference and service center for all aspects of primate research. The DPZ is one of the 96 research and infrastructure facilities of the Leibniz Association.
Media Contact
Susanne Diederich
[email protected]
Related Journal Article
http://dx.