Credit: KAIST
Researchers suggest that modulation of local CO2 concentration improves the selectivity, conversion rate, and electrode stability, and shed a new light on the electrochemical CO2 reduction technology for controlling emissions at a low cost.
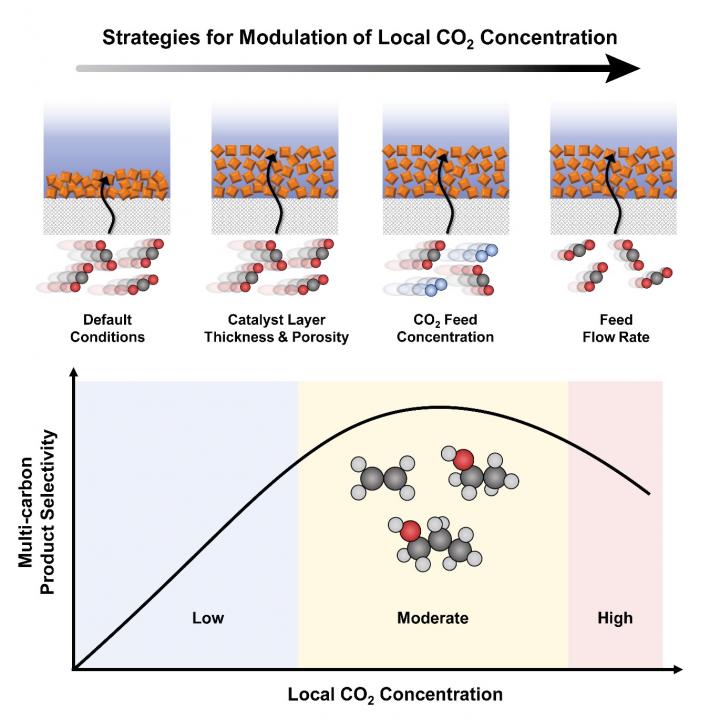
A KAIST research team presented three novel approaches for modulating local carbon dioxide (CO2) concentration in gas-diffusion electrode (GDE)-based flow electrolyzers. Their study also empirically demonstrated that providing a moderate local CO2 concentration is effective in promoting Carbon-Carbon (C-C) coupling reactions toward the production of multi-carbon molecules. This work, featured in the May 20th issue of Joule, serves as a rational guide to tune CO2 mass transport for the optimal production of valuable multi-carbon products.
Amid global efforts to reduce and recycle anthropogenic CO2 emissions, CO2 electrolysis holds great promise for converting CO2 into useful chemicals that were traditionally derived from fossil fuels. Many researches have been attempting to improve the selectivity of CO2 for commercially and industrially high-value multi-carbon products such as ethylene, ethanol, and 1-propanol, due to their high energy density and large market size.
In order to achieve the highly-selective conversion of CO2 into valuable multi-carbon products, past studies have focused on the design of catalysts and the tuning of local environment related to pH, cations, and molecular additives.
Conventional CO2 electrolytic systems relied heavily on an alkaline electrolyte that is often consumed in large quantities when reacting with CO2, and thus led to an increase in the operational costs. Moreover, the life span of a catalyst electrode was short, due to its inherent chemical reactivity.
In their recent study, a group of KAIST researchers led by Professor Jihun Oh from the Department of Materials Science and Engineering reported that the local CO2 concentration has been an overlooked factor that largely affects the selectivity toward multi-carbon products.
Professor Oh and his researchers Dr. Ying Chuan Tan, Hakhyeon Song, and Kelvin Berm Lee proposed that there is an intimate relation between local CO2 and multi-carbon product selectivity during electrochemical CO2 reduction reactions. The team employed the mass-transport modeling of a GDE-based flow electrolyzer that utilizes copper oxide (Cu2O) nanoparticles as model catalysts. They then identified and applied three approaches to modulate the local CO2 concentration within a GDE-based electrolytic system, including 1) controlling the catalyst layer structure, 2) CO2 feed concentration, and 3) feed flow rate.
Contrary to common intuition, the study showed that providing a maximum CO2 transport leads to suboptimal multi-carbon product faradaic efficiency. Instead, by restricting and providing a moderate local CO2 concentration, C-C coupling can be significantly enhanced.
The researchers demonstrated experimentally that the selectivity rate increased from 25.4% to 61.9%, and from 5.9% to 22.6% for the CO2 conversion rate. When a cheap milder near-neutral electrolyte was used, the stability of the CO2 electrolytic system improved to a great extent, allowing over 10 hours of steady selective production of multi-carbon products.
Dr. Tan, the lead author of the paper, said, “Our research clearly revealed that the optimization of the local CO2 concentration is the key to maximizing the efficiency of converting CO2 into high-value multi-carbon products.”
Professor Oh added, “This finding is expected to deliver new insights to the research community that variables affecting local CO2 concentration are also influential factors in the electrochemical CO2 reduction reaction performance. My colleagues and I hope that our study becomes a cornerstone for related technologies and their industrial applications.”
###
This work was supported by the Korean Ministry of Science and ICT (MSIT) Creative Materials Discovery Program.
Media Contact
Younghye Cho
[email protected]
Original Source
http://news.
Related Journal Article
http://dx.




