Mitophagy reporter mouse could provide cues for future therapies and rehabilitation strategies
Niigata, Japan – The loss of muscle tissue – referred to as muscle atrophy in medical terms – can occur as a result of lack of physical activity for an extended period of time; aging; alcohol-associated myopathy – a pain and weakness in muscles due to excessive drinking over long periods of time; burns; injuries; malnutrition; spinal cord or peripheral nerve injuries; stroke; and long-term corticosteroid therapy. While muscle atrophy due to disuse is well known and studied, the underlying cellular mechanisms, particularly the status of mitochondrial degradation by mitophagy during disuse-induced muscle atrophy has been a subject of debate among cellular physiologists as mitochondria are abundant in skeletal muscles.
Mitophagy is a cellular quality control mechanism by which cells break down damaged or dysfunctional subcellular organelles called mitochondria. While mitochondria play a crucial role in cellular energy production among other cellular homeostatic functions, mitochondrial release of reactive oxygen species (ROS) during disuse-induced muscle atrophy has previously been reported. It is thought that ROS oxidizes proteins, lipids, and nucleic acids, leading to decreased protein production and eventually muscle atrophy.
A group of researchers from the Department of Cellular Physiology, Graduate School of Medical and Dental Sciences, Niigata University in collaboration with Taisho Pharmaceutical Co. Ltd. and the National Institute of Quantum and Radiological Science and Technology in Japan have developed a new fluorescent reporter mouse line to detect changes in mitophagy activity that could improve treatment strategies and possibly facilitate strategies to reverse muscle atrophy induced by disuse.
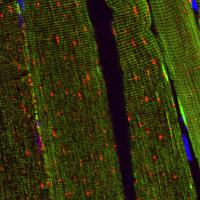
In a mouse model study, the authors devised the in vivo fluorescent approach utilizing a dual mCherry/EGFP (red/green) reporter to enable the visualization of mitochondrial changes in muscle tissue sections from mice whose hind limbs were immobilized (hindlimb IM) (pictured, right). They followed the existence of cytosolic mitochondria with co-expression of green EGFP and red mCherry proteins, however when mitochondria are delivered into lysosomes forming mitolysosomes under mitophagy conditions, the green EGFP protein is quenched and degraded allowing direct analysis of mitophagy activity in vivo.
While there are previous studies where other mitophagy analysis methods have been used, Dr. Keiichi Inoue and Professor Tomotake Kanki have elaborated on how their method is different, saying “Our new reporter mouse line enables to directly analyze the mitophagy activity in vivo. This is more advantageous than the previous indirect methods based on the expression of mitophagy markers as those genes are unspecific to the mitophagy induction.” This dual fluorescent reporter system allowed the direct and specific monitoring of mitophagy activity in vivo.
The authors in their quest to spotlight changes in mitophagy in relation to disuse-induced atrophying muscle assessed the mitophagy activity using new reporter mice. They reported increased levels of mitophagy activity as well as ROS levels in atrophic soleus muscles following a 14-day hindlimb immobilization, revealing that muscle disuse increased mitophagy activity in skeletal muscle.
The study highlights mitophagy as a potential therapeutic target for disuse-induced muscle atrophy. Furthermore, as muscle atrophy is accompanied by physiological aging or some pathological states, such as myopathy or neuropathy, this new model will be helpful to understand and prevent muscle atrophy following those changes.
“We will further reveal the dynamics of mitophagy in other physiological and pathological consequences,” they further added.
###
The article “Mitophagy reporter mouse analysis reveals increased mitophagy activity in disuse-induced muscle atrophy” was published in Journal of Cellular Physiology.
https:/
Media Contact
Tomotake Kanki
[email protected]
Original Source
https:/
Related Journal Article
http://dx.