Adding a flux during the synthesis of oxyhydrides is a promising strategy to obtain a pure, homogenous product, reveal scientists from Tokyo Tech. An SrCl2 flux promoted the melting of a part of reactants and facilitated their diffusion of reactants, which proved to be the key to producing highly pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides in high-pressure and high-temperature reactions. These compounds have potential as catalysts and as electrode materials for lithium-ion batteries.
Credit: Associate Professor Takafumi Yamamoto
Adding a flux during the synthesis of oxyhydrides is a promising strategy to obtain a pure, homogenous product, reveal scientists from Tokyo Tech. An SrCl2 flux promoted the melting of a part of reactants and facilitated their diffusion of reactants, which proved to be the key to producing highly pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides in high-pressure and high-temperature reactions. These compounds have potential as catalysts and as electrode materials for lithium-ion batteries.
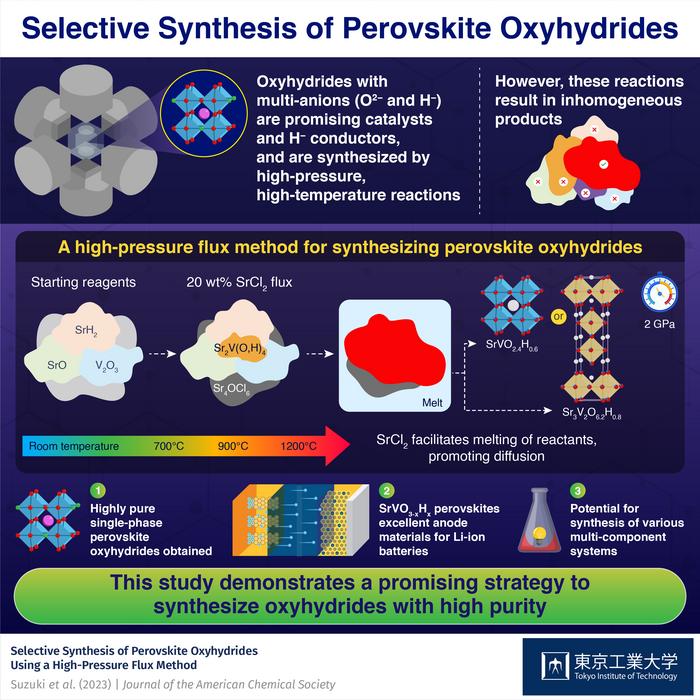
Perovskite oxyhydrides containing oxide (O2–) and hydride (H–) anions are promising compounds with applications in catalytic systems and batteries. Unfortunately, synthesizing oxyhydrides is usually quite challenging, mainly due to the highly reactive nature of H– anions.
It was known that high-pressure and high-temperature reactions are effective to synthesize oxyhydrides. For example, Sr2VO4–xHx perovskite can be synthesized directly from oxide and hydride precursors in high-pressure and high-temperature reactions. A key advantage of these reactions is that the H– content in the final product can be tuned by adjusting the composition and ratio of the precursors. This essentially means that the electronic and magnetic properties of the product are also customizable. Unlike Sr2VO4–xHx, synthesizing SrVO3–xHx has proven much more difficult, since the necessary high-pressure and high-temperature reactions lead to the formation of several impurities and inhomogeneous products, mainly due to insufficient diffusion of the solid components.
In a recent study published in Journal of American Chemical Society, a research team led by Associate Professor Takafumi Yamamoto from the Institute of Innovative Research at Tokyo Institute of Technology (Tokyo Tech) found a solution to this problem. They developed a novel approach to synthesize highly pure SrVO2.4H0.6 and Sr3V2O6.2H0.8, two new perovskite oxyhydrides. This study was conducted as part of a collaborative research project with the National Institutes for Quantum Science and Technology, Japan.
The researchers started with SrO, SrH2, and V2O3, and added SrCl2 to these reactants. They observed the differences in the composition of samples prepared under different conditions using a technique called in-situ synchrotron X-ray diffraction, shedding light on the role of SrCl2 in the reaction. It acted as a flux at a high temperature of 1200 ℃ and a high pressure of 2 GPa, facilitating the melting and dissolution of a part of reactants, thus promoting diffusion. Consequently, the researchers managed to suppress the development of inhomogeneous products that typically appear due to insufficient diffusion, obtaining highly pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides.
Additionally, the team analyzed the electrochemical properties of the prepared perovskite oxyhydrides as an electrode material. “With low working potential, excellent reversibility, and high-rate characteristics, SrVO3–xHx could be suitable as a negative electrode for lithium-ion batteries, a first for oxyhydrides,” highlights Dr. Yamamoto.
Overall, using a flux to boost the desired reaction pathways in high-pressure and high-temperature reactions could be a powerful strategy to unlock a plethora of new compounds beyond perovskite oxyhydrides. Dr. Yamamoto remarks: “The proposed synthesis approach would also be effective in the synthesis of various types of multi-component systems.”
Let us hope that these findings lead to new breakthroughs in energy storage and other areas of applied chemistry!
###
Dr. Takafumi Yamamoto | Yamamoto Group
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
https://www.titech.ac.jp/english/
Journal
Journal of the American Chemical Society
DOI
10.1021/jacs.3c02240
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Selective Synthesis of Perovskite Oxyhydrides Using a High-Pressure Flux Method
Article Publication Date
25-Jul-2023




