Credit: ©Laboratoire Bourquin – UNIFR/UNIGE
The use of nanoparticles — small, virus-sized elements developed under laboratory conditions — is increasingly widespread in the world of biomedicine. This rapidly-evolving technology offers hope for many medical applications, whether for diagnosis or therapies. In oncology, for example, the growing body of research suggests that, thanks to nanoparticles, treatment will soon become more precise, more effective and less painful for the patients. However, the way nanoparticles interact with the immune system remained unclear and unpredictable until recently, restricting their potential medical use. Today, researchers from the universities of Geneva (UNIGE) and Fribourg (UNIFR), Switzerland, are close to solving the problem: they have devised a rapid screening method to select the most promising nanoparticles, thereby fast-tracking the development of future treatments. In less than a week, they are able to determine whether nanoparticles are compatible or not with the human body — an analysis that previously required several months of work. This discovery, which is described in the journal Nanoscale, may well lead to the swift, safe and less expensive development of nanotechnology applied to medicine.
Nanoparticles measure between 1 and 100 nanometres, approximately the size of a virus. Their very minuteness means that they have the potential to be used in a wide range of medical applications: serving as markers for diagnosis, for example, or delivering therapeutic molecules to the exact spot in the body where the drug is intended to act. However, before being applied to the medical field, nanoparticles must prove (i) that they are safe for the human body and (ii) that they are capable of bypassing the immune system so they can have an effect. "Researchers can spend years developing a nanoparticle, without knowing what impact it will have on a living organism," explains Carole Bourquin, professor in the medicine and science faculties at UNIGE and project leader. "So there was a real need to design an effective screening method that could be implemented at the beginning of the development process. Indeed, if the nanoparticles aren't compatible, several years of research were simply thrown away.»
Macrophages orchestrate the immune response
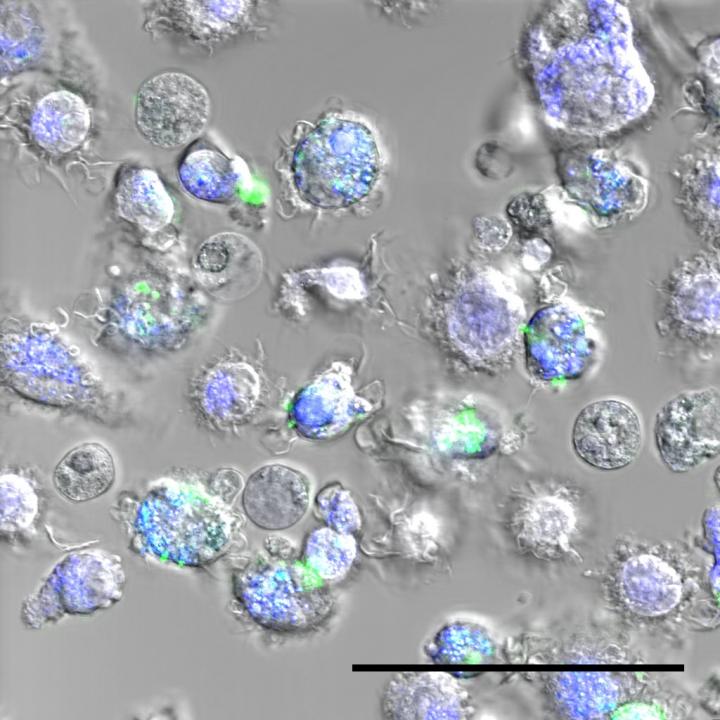
When a foreign element — any foreign element — enters the body, the immune system is activated. Macrophages are always found on the front line, large cells that «ingest» invaders and trigger the immune response. Nanoparticles are no exception to this rule. The way macrophages react to the nanoparticle under investigation then predicts the biocompatibility of the product. «When you begin to develop a new particle, it's very difficult to ensure that the recipe is exactly the same every time,» points out Inès Mottas, the first author. «If we test different batches, the results may differ. Hence our idea of finding a way to test the three parameters simultaneously — and on the same sample — to establish the product's biocompatibility: its toxicity, its ability to activate the immune system, and the capacity of the macrophages to ingest them.»
The ideal medical nanoparticle should therefore not be toxic (it should not kill the cells); should not be entirely ingested by the macrophages (so that it retains its power to act); and should limit the activation of the immune system (to avoid adverse side-effects).
Evaluating the three key elements simultaneously
Until now, evaluating the biocompatibility of nanomaterials was a laborious task that took several months and posed reproducibility problems, since not all the tests were performed on the same batch of particles. Professor Bourquin and her team used flow cytometry to reach a diagnosis on the three essential elements in a safe and standardised manner, and in record time. «The macrophages are brought into contact with the nanoparticles for 24 hours, and are then passed in front of the laser beams. The fluorescence emitted by the macrophages makes it possible to count them and characterise their activation levels. Since the particles themselves are fluorescent, we can also measure the amount ingested by the macrophages. Our process means we can test the three elements simultaneously, and we only need a very small amount of particles,» continues Mottas. «We can obtain a comprehensive diagnosis of the nanoparticle submitted to us in two or three days.»
The method devised in Geneva and Freiburg is part of the work carried out within the National Centres of Competence in Research (NCCR) "Bio-Inspired Materials", and is already a great success with scientists striving to develop new particles. It focuses their work by enabling them to select the most promising particles quickly. As well as having a financial impact on the cost of research, this new approach also limits the use of animal testing. Furthermore, it is opening the door to the increasingly personalized treatment of certain pathologies. For example, by testing the nanoparticles on tumour cells isolated from a particular patient, it should theoretically be possible to identify the most effective treatment. Only time will tell whether this hypothesis is backed up in practice.
###
Media Contact
Carole Bourquin
[email protected]
41-223-799-071
@UNIGEnews
http://www.unige.ch





