Credit: Dr. WU Beili
Class B G protein-coupled receptors (GPCRs) exert essential action in hormonal homeostasis and are important therapeutic targets for a variety of diseases including metabolic disorders such as type 2 diabetes. These receptors consist of an extracellular domain (ECD) and a transmembrane domain (TMD), both of which are required to interact with their cognate peptide ligands and to regulate downstream signal transduction. Due to difficulties in high-quality protein preparation, determination of the structure of full-length class B GPCRs remains a challenge, thus limiting the understanding of molecular mechanisms of receptor action.
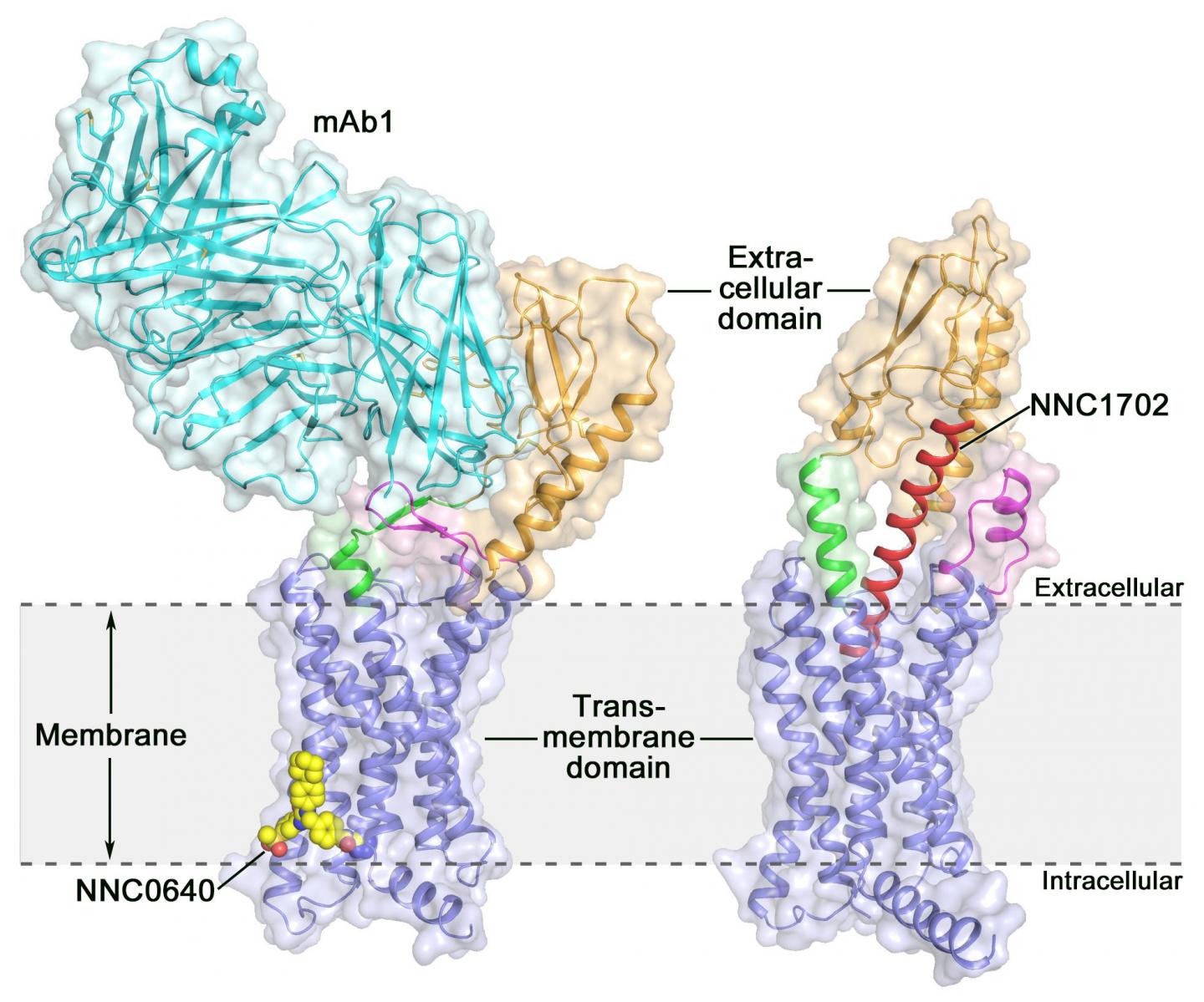
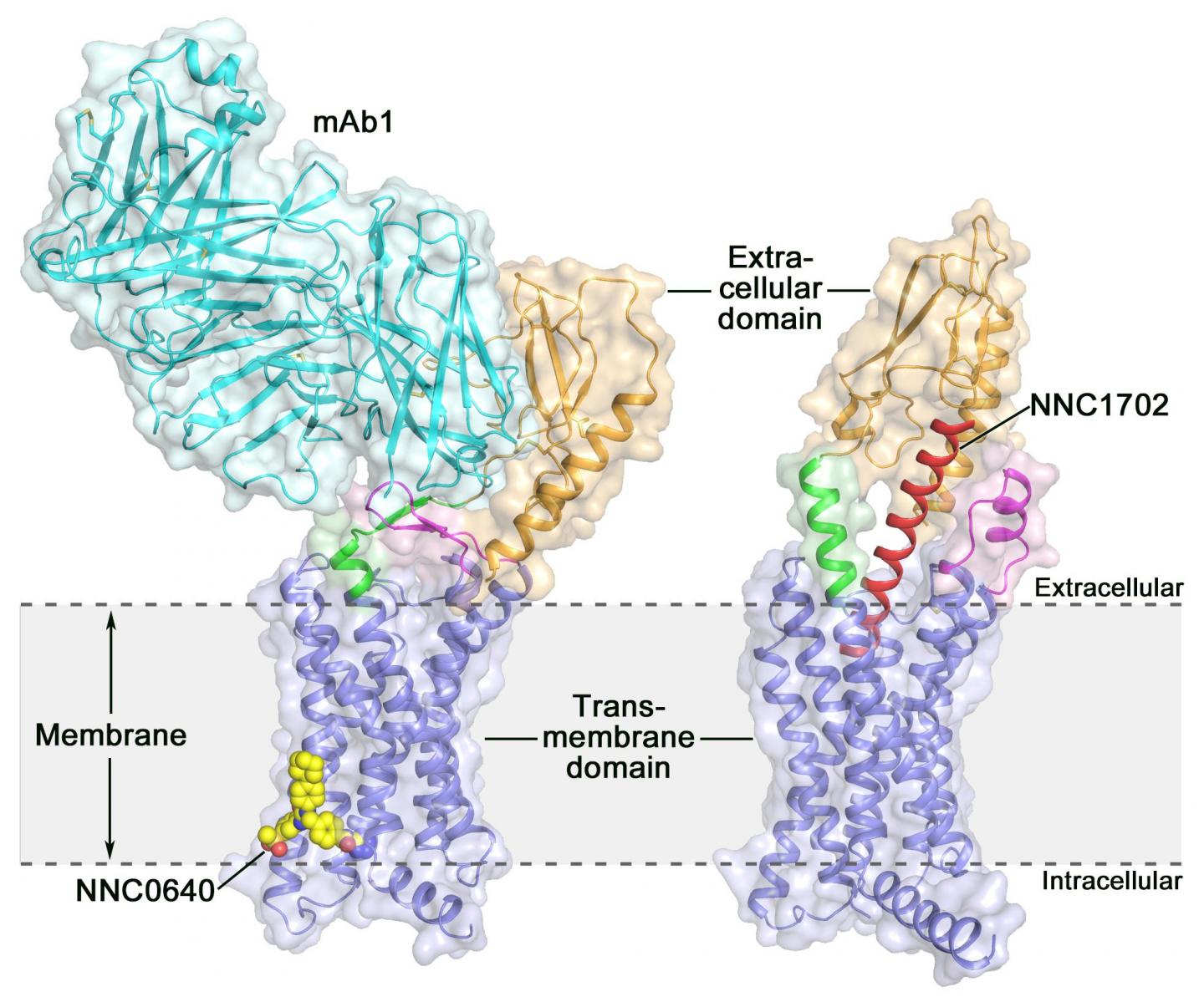
Activation of the human glucagon receptor (GCGR) by its endogenous ligand glucagon triggers the release of glucose from the liver during fasting, making it a potential drug target for type 2 diabetes. Last year, a group of scientists at the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences determined the crystal structure of the full-length GCGR bound to a negative allosteric modulator NNC0640 and an inhibitory antibody mAb1, thus providing for the first time a clear picture of a full-length class B GPCR at high resolution.
Recently, scientists at SIMM determined the crystal structure of GCGR in complex with a glucagon analogue and partial agonist NNC1702. This structure reveals, for the first time, the molecular details of a class B GPCR binding to its peptide ligand at high resolution and unexpectedly discloses the structural complexity that governs receptor activation, thereby greatly expanding the understanding of class B GPCR signal transduction. The study was published in Nature.
This study offers some valuable insights into the activation mechanism of GCGR. The most exciting finding is that the linker region connecting the ECD and TMD of the receptor, termed the "stalk," and the first extracellular loop undergo significant conformational changes in their secondary structures in the peptide-bound GCGR structure compared to the previously determined non-peptide-bound structure. This leads to a marked change in the relative orientation between the ECD and TMD of the receptor to accommodate peptide binding and initiate receptor activation.
Furthermore, the stalk may modulate receptor activity by facilitating conformational movements of the receptor TMD. "It is amazing to observe how the stalk region plays such an important role in regulating receptor function, although it only contains 12 amino acids," said SIMM professor Dr. ZHAO Qiang. "This has never been observed in previously solved GPCR structural studies. It significantly deepens the knowledge about class B GPCR signaling mechanisms."
Based on the structure of GCGR-NNC1702 complex, the researchers performed a series of functional studies using techniques such as competitive ligand binding, cell signaling, molecular dynamics simulations and double electron-electron resonance spectroscopy. The results support the GCGR structure and confirm the conformational alterations of the receptor in different functional states.
"The newly solved GCGR structure provides the most accurate template to date for drug design targeting GCGR, which offers new opportunities in drug discovery for treating type 2 diabetes," said team leader and SIMM professor Dr. WU Beili.
###
This study was a collaboration work conducted by researchers from SIMM, Fudan University, the iHuman Institute of ShanghaiTech University, Novo Nordisk, University of Toronto, and GPCR Consortium.
Media Contact
WU Beili
[email protected]
http://english.cas.cn/