Credit: Discher Lab.
One reason cancer is so difficult to treat is that it avoids detection by the body. Agents of the immune system are constantly checking the surfaces of cells for chemical signals that say they belong, but cancer cells express the same chemical signals as healthy ones. Without a way for the immune system to tell the difference, little stands in the way of cancer taking over.
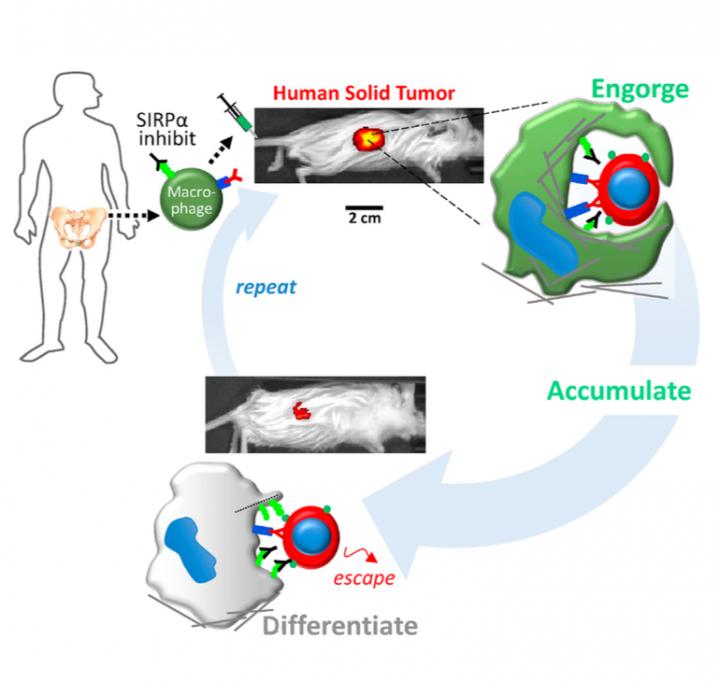
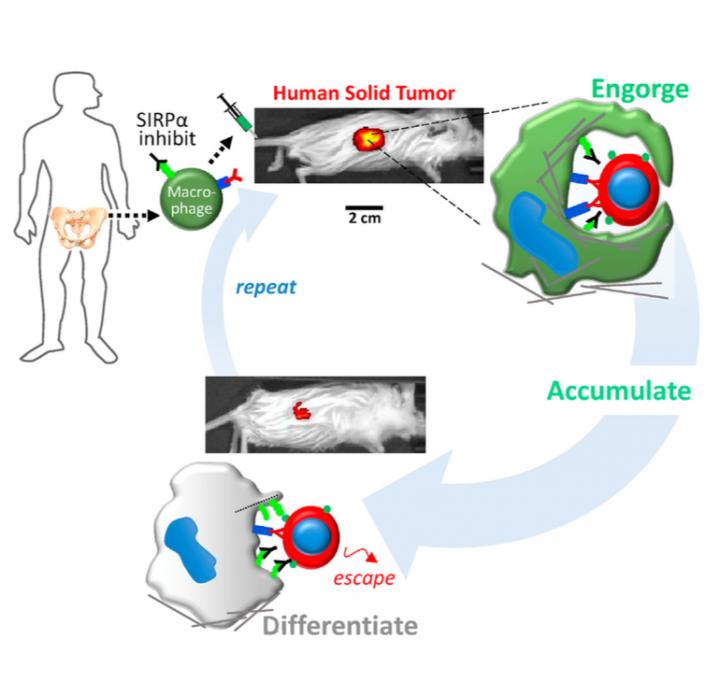
Now, researchers at the University of Pennsylvania School of Engineering and Applied Science and Penn's Perelman School of Medicine and Physical Sciences Oncology Center have learned how to re-engineer macrophages, the "first responders" of the immune system, so that they can distinguish between healthy and cancerous cells.
Armed with this ability, the engineered cells were able to circulate through the body of a mouse, invade solid tumors and specifically engulf human cancer cells therein. This represents a potential advance in cancer immunotherapy, which has so far found the greatest success in treating "liquid" tumors associated with blood cancers.
The research was led by Dennis E. Discher, the Robert D. Bent Professor in Penn Engineering's Department of Chemical and Bimolecular Engineering, and Cory Alvey, a graduate student in his lab from the Department of Pharmacology in Penn Medicine. Other Discher lab members who contributed to the work were Kyle Spinler, Jerome Irianto, Charlotte Pfeifer, Brandon Hayes, Yuntao Xia, Sangkyun Cho, Dave Dingal , Jake Hsu, Lucas Smith and Manu Tewari.
The work was published in the journal Current Biology.
Immune cells called macrophages are the body's first responders; they remove diseased or foreign cells from the body by devouring and digesting them. Macrophages should also destroy cancerous cells, but, because they arise from healthy cells, the former are protected by nearly all of the same mechanisms that keep the latter from being attacked by the immune system.
The Discher lab has expertise with mimicking these protective mechanisms on healthy cells, but now they are targeting the proteins on macrophages that respond to those signals.
"Our new approach takes young and aggressive macrophages from the bone marrow of a human donor and removes a key safeguard that cancer cells have co-opted to prevent them from being engulfed," Alvey said. "Combined with cancer-specific targeting antibodies, these engineered macrophages swarm into solid tumors and rapidly drive regression of human tumors without any measurable toxicity."
Immune cell therapies using engineered T-cells have recently emerged as successful treatments for some blood cancers, which are referred to as "liquid" tumors. Tumors in other tissues are generally more solid, which can physically impede the ability of T-cells to penetrate into the mass of the tumor.
Macrophages readily infiltrate diseased and damaged tissues, including tumors. As such, macrophage-based cancer therapies were investigated decades ago. While they were found to be safe in patients, they were not effective in destroying cancerous cells. It is now understood that such macrophages received the same "don't eat me" signal from both healthy and cancerous cells.
The Discher Lab has since shown that a protein on human cells called CD47 functions as a "marker of self" by interacting with a protein on the surface of macrophages called SIRPA. When SIRPA contacts CD47 on any other cell, it serves as a safeguard that prevents the macrophage from engulfing the other cell, even if it's cancerous. With that in mind, the researchers thought that controlling this protein might revitalize macrophage-based cell therapies.
Injections of antibody molecules that block CD47 from interacting with SIRPA are already being tried in the clinic based on observations of some reduction in the sizes of tumors in mouse models. However, such molecular treatments reproducibly cause rapid loss of many circulating blood cells, as macrophages now attack some healthy cells as well. In addition to causing anemia, some mice with depleted CD47 die from auto-immune disease.
To get around these safety concerns and to potentially maximize therapeutic effects on tumors, Alvey and colleagues took fresh, young macrophages from human donors as well as mouse donors and directly blocked their SIRPA. They also injected various antibodies that bind to cancer cells, which help to activate macrophages that might enter the tumor.
"The big surprise," Discher said, "is that injected macrophages circulate all around the body but accumulate only within the tumors where they engorge on cancer cells."
After two injections, cancer cells were depleted 100-fold from tumors the size of a dime, and tumors regressed 80 percent in size. Importantly, blood cells were unaffected by the treatments, which suggests that this approach is safe.
"Safety thus far is likely a consequence of both the relatively small number of engineered macrophages that are injected and their sequestration into the tumors, away from most healthy cells," Discher said.
New biological mechanisms were uncovered by the study, including the observation that the injected macrophages which accumulate in the tumor stop engorging on cancer cells after about a week. Further injections, however, safely drive tumor regression.
"The first phase of clinical trials are tests of human safety, so this is a promising start," Alvey said. "The potency of these engineered macrophages is relatively clear, but the crucial issue is how to maximize the anti-cancer effects while minimizing side effects, namely the engulfment of normal cells."
Ongoing studies from the Discher lab are focused on finding that sweet spot with additional tumor models, as well as on how to engineer macrophages for longer-lasting effect.
###
The research was supported by the National Institutes of Health through grants U54-CA193417, R01-HL124106 ,K99 AR067867 and CA016520 and by the National Science Foundation through Grant DMR-1120901.
Media Contact
Evan Lerner
[email protected]
215-573-6604
@Penn
http://www.upenn.edu/pennnews
Original Source
https://news.upenn.edu/news/penn-researchers-engineer-macrophages-engulf-cancer-cells-solid-tumors http://dx.doi.org/10.1016/j.cub.2017.06.005