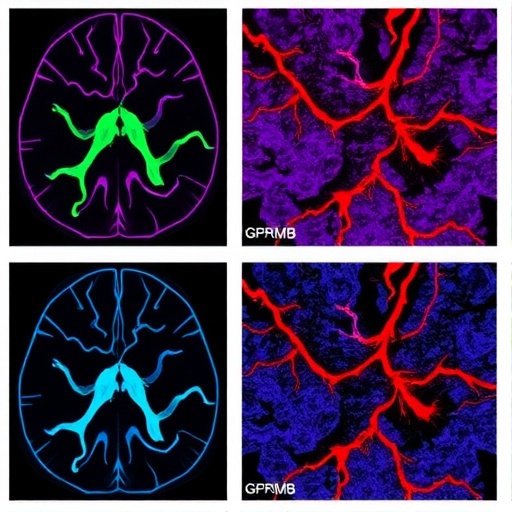
In a groundbreaking study, a research team led by Du, Long, and Li has unveiled the intricate relationship between spatially-reprogrammed GPNMB+ macrophages and COL6A3+ fibroblasts in the context of vascular fibrosis associated with glioblastoma. This research, featured in the prestigious journal “Genome Medicine,” sheds light on the cellular interactions that exacerbate tumor progression in one of the most aggressive forms of brain cancer.
Glioblastoma multiforme (GBM) is notorious for its poor prognosis and highly invasive nature, leading to substantial morbidity in affected individuals. The complexity of this malignancy is further underscored by its microenvironment, comprised of various cell types, including immune cells, stromal cells, and extracellular matrix components. The interplay between these elements is crucial for tumor growth and metastasis, presenting a fertile ground for research aimed at unraveling the mechanisms behind GBM’s resilience.
The study identifies GPNMB+ macrophages as pivotal players in the tumor microenvironment. These cells are derived from the reprogramming of monocytes and exhibit distinct phenotypic and functional characteristics that contribute to the fibrotic milieu surrounding the tumor. The spatial reprogramming of these macrophages is triggered by the local cues provided by the glioblastoma microenvironment, driving their transformation into a pro-fibrotic phenotype.
Importantly, the interaction between GPNMB+ macrophages and COL6A3+ fibroblasts plays a critical role in enhancing vascular fibrosis. COL6A3, a collagen type often associated with tissue repair and fibrosis, is secreted by fibroblasts and contributes to the structural integrity of the tumor microenvironment. The study reveals that GPNMB+ macrophages promote COL6A3 expression in fibroblasts, thereby amplifying the fibrotic response and facilitating tumor growth.
The implications of these findings are immense, as they not only provide insight into the cellular dynamics of glioblastoma but also highlight potential therapeutic targets. By understanding the signaling pathways involved in the macrophage-fibroblast interaction, researchers can devise strategies to disrupt this pro-fibrotic loop. Such interventions could hinder glioblastoma progression and improve patient outcomes, making a substantial impact on the treatment landscape of this challenging disease.
Furthermore, the study details the molecular pathways activated in GPNMB+ macrophages upon interaction with COL6A3+ fibroblasts. These include pro-inflammatory cytokines and growth factors that perpetuate a cycle of inflammation and fibrosis. The identification of these pathways opens new avenues for pharmacological intervention, aimed at disrupting the cytokine signaling cascade responsible for enhancing vascular fibrosis.
As glioblastoma continues to pose significant treatment challenges, the findings from this research underscore the importance of targeting the tumor microenvironment. Historical approaches have focused primarily on direct cytotoxic strategies against tumor cells. However, by shifting the focus towards the supporting cellular infrastructure, researchers can develop a more holistic approach to cancer therapy.
In addition to therapeutic implications, this research calls for further investigation into the heterogeneity of macrophage populations within glioblastoma. Understanding how different macrophage subsets contribute to tumor pathology can enhance our grasp of intra-tumoral dynamics and lead to more personalized medicine approaches tailored to individual patient profiles.
In summary, the intricate relationship between GPNMB+ macrophages and COL6A3+ fibroblasts reveals a sophisticated network that fuels glioblastoma progression through enhanced vascular fibrosis. This study marks a significant step forward in our understanding of the molecular underpinnings of glioblastoma, offering hope for novel therapeutic strategies aimed at curbing this devastating disease.
Future research should focus on exploring the therapeutic feasibility of targeting GPNMB+ macrophages in glioblastoma. The potential to modify the macrophage phenotype to a more anti-tumorigenic state may prove pivotal in improving patient prognosis. Moreover, understanding how to manipulate COL6A3 expression in fibroblasts could reveal additional targets for intervention.
The findings also suggest a need for clinical trials examining agents that can mitigate the effects of GPNMB+ macrophages or COL6A3+ fibroblasts. Such trials could lead to innovative therapies that could transform the management of glioblastoma and extend survival rates for patients battling this formidable foe.
Ultimately, this study not only deepens our understanding of glioblastoma biology but also invites scientists and clinicians alike to explore collaborative efforts aimed at deciphering the complexities of tumor-stromal interactions. Only through understanding the full landscape of glioblastoma can effective therapies be realized.
In conclusion, the research conducted by Du and colleagues represents a critical advancement in uncovering the malignant strategies of glioblastoma, focusing on the significance of immune-transformed macrophages and the fibrotic landscape they influence. As we delve deeper into the myriad of interactions that support tumor growth, the quest for effective treatment options remains paramount.
Subject of Research: Glioblastoma
Article Title: Spatial-reprogramming derived GPNMB+ macrophages interact with COL6A3+ fibroblasts to enhance vascular fibrosis in glioblastoma.
Article References:
Du, Y., Long, X., Li, X. et al. Spatial-reprogramming derived GPNMB+ macrophages interact with COL6A3+ fibroblasts to enhance vascular fibrosis in glioblastoma.
Genome Med 17, 136 (2025). https://doi.org/10.1186/s13073-025-01553-2
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s13073-025-01553-2
Keywords: Glioblastoma, GPNMB, COL6A3, macrophages, fibroblasts, vascular fibrosis, tumor microenvironment.
Tags: COL6A3 fibroblasts in glioblastomaextracellular matrix in brain tumorsfibrotic response in tumorsglioblastoma multiforme characteristicsglioblastoma research advancementsGPNMB macrophages in glioblastomaimmune cell contributions to cancermechanisms of glioblastoma progressionrole of macrophages in tumor microenvironmentspatial reprogramming of immune cellstumor microenvironment interactionsvascular fibrosis in brain cancer