Researchers are continuing to make strides in the field of immunotherapy, particularly focusing on the treatment of relapsed or refractory B-cell lymphomas. Among the innovative approaches, the combination of chimeric antigen receptor T-cell (CAR-T) therapy and bispecific antibodies (BsAbs) has emerged as a promising strategy, showing potential for enhanced efficacy in therapeutic outcomes. This integrated approach targets both the tumor cells and the T-cells within the immune system, creating a more robust defense against malignancies.
The mechanism by which CAR-T therapies operate has been the subject of extensive research, yet this study delves into a lesser-explored territory: the roles of bystander T-cells within the CAR-T product. In essence, CAR-T cell products are not solely composed of CAR-engineered cells; they also contain a subset of CAR-negative bystander T-cells that may provide synergistic effects. These bystander T-cells are often overlooked in favor of the engineered CAR-T cells, but emerging evidence suggests that they may play a critical role in amplifying the immune response against tumors once the cytokine environment is favorable.
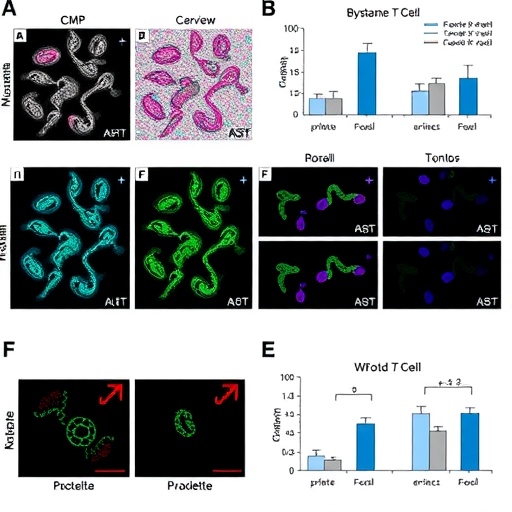
To investigate this phenomenon, the researchers chronologically assessed T-cell clones and their memory characteristics within peripheral blood mononuclear cells (PBMCs) and lymph nodes collected from patients treated sequentially with a CAR-T therapy targeting CD19 followed by a CD20 x CD3 BsAb. The longitudinal aspect of this research provides unique insights into how T-cell populations evolve following such therapies, shedding light on the complex immunological dynamics at play.
.adsslot_IGWTAo8COk{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_IGWTAo8COk{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_IGWTAo8COk{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Notably, the study revealed that the memory-formed T-cell population significantly expanded after CAR-T therapy, particularly in both the peripheral blood and lymph nodes. The expansion of these memory T-cells indicates not only a successful engagement with the tumor but also suggests potential for long-lasting immunity. As anticipated, these memory T-cells showed further proliferation after the administration of the BsAb, indicating that the sequential application of therapies can create a compounded effect on T-cell activation and mobilization.
An unexpected yet fascinating finding from the analysis was the identification of product-derived CAR-negative CD8+ T-cells that, following CAR-T therapy, not only proliferated but also infiltrated relapsed lymph nodes. This expansion of bystander T-cells post-treatment suggests an orchestrated immune response that enhances the overall therapeutic effect, particularly when grappling with the challenge of tumor relapse. This insight underscores the necessity to reevaluate the significance of these bystander T-cells in the context of CAR-T therapy, especially in combination therapies.
Moreover, the concept of leveraging the bystander effect carries implications for the development of more effective therapeutic strategies. By understanding the factors that contribute to the activation and expansion of these T-cells, researchers can devise methodologies to enhance their contribution to the immune response. This could lead to improved outcomes for patients experiencing treatment-resistant cancer types, where conventional strategies may fall short.
Researchers advocate for a refined perspective on T-cell therapy that encompasses both CAR-engineered and bystander cells, encouraging future studies to explore their interplay and collective impact on treatment outcomes. The integration of these insights can forge pathways towards more holistic therapeutic modalities that maximize the potential of existing and novel immunotherapeutic agents, thereby straddling the line between efficacy and safety.
The findings of this study suggest a paradigm shift towards utilizing the full arsenal of the immune response against tumors. The collaborative dynamics between CAR-T cells and bystander T-cells present an opportunity to capitalize on all available resources within the immune system, potentially yielding higher rates of tumor remission and improved patient prognoses. Understanding how to manipulate these interactions can revolutionize treatment regimens.
In summary, the significance of bystander T-cells in the context of CAR-T therapy is just beginning to unfold. This research paves the way for further inquiry into the mechanisms of action, integration, and activation of these cells. As scientists continue to dissect the intricate immunological pathways involved, the potential for innovative treatment protocols that blend CAR-T therapy with BsAbs and other modalities becomes increasingly apparent.
Looking forward, the immuno-oncology community is poised to witness shifts in both clinical practice and research focus as the implications of this study are fully realized. The integration of bystander T-cells into the therapeutic narrative not only holds promise for enhanced efficacy but also calls for a reevaluation of how therapies are designed, monitored, and optimized. This evolution in understanding may very well define the next chapter in the battle against B-cell malignancies, showcasing the remarkable adaptability and complexity of our immune responses.
The potential therapeutic avenues suggested by this research will encourage ongoing dialogues among oncologists, immunologists, and researchers, fostering collaborative efforts that aim to bridge the gap between basic science and clinical application. As this field progresses, the understanding of T-cell biology will continue to deepen, driving innovations that could transform patient care standards.
With the insights gained from this study, there is hope for exciting advancements in treatment options, ultimately benefiting patients facing daunting cancer challenges. As the research community rallies to harness these discoveries, the era of personalized cancer therapy continues to evolve, promising a brighter future for those impacted by this often devastating disease.
Subject of Research: Investigation of bystander T-cells in CAR-T therapy and their role in enhancing therapeutic effects when used in conjunction with bispecific antibodies.
Article Title: The Role of Bystander T-Cells in Enhancing CAR-T Therapy Efficacy
News Publication Date: Not provided
Web References: Not provided
References: Not provided
Image Credits: Junichi Kato, Tatsuya Konishi, Toshiki Ochi, Katsuto Takenaka, Ehime University
Keywords
Cancer immunotherapy, CAR-T therapy, bispecific antibodies, B-cell lymphomas, T-cell biology, cytokine release, immunological memory.
Tags: bispecific antibodies in immunotherapybystander T cells in cancer therapyCAR-T therapy and B-cell lymphomascombination therapies for cancer treatmentcytokine environment and immune responseenhancing antitumor efficacyimmune response amplification mechanismsimmunotherapy advancements for relapsed lymphomamemory characteristics of T-cell clonesperipheral blood mononuclear cells analysisrole of bystander T cells in CAR-T therapysynergistic effects of T-cell subsets