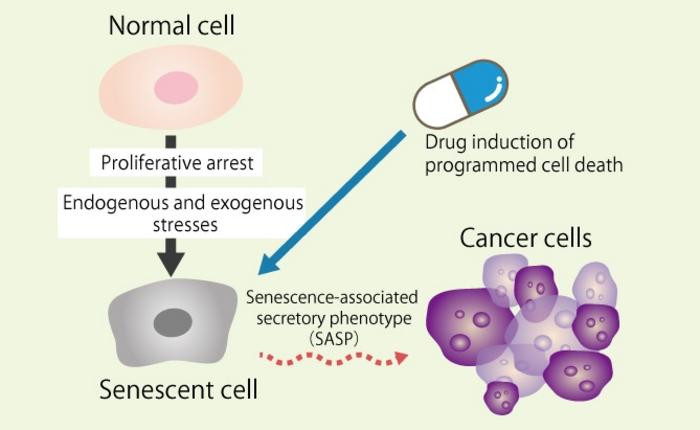
As the global population ages, understanding the biological processes associated with senescence becomes increasingly critical in the fight against cancer and age-related diseases. Cellular senescence, a state where cells lose the ability to divide, plays a significant role in aging and the development of various pathologies. One of the key features of senescent cells is their distinct secretory phenotype, commonly referred to as the senescence-associated secretory phenotype (SASP). This phenotype is characterized by the secretion of pro-inflammatory factors, growth factors, and proteases that can contribute to tumorigenesis and foster a tumor-promoting environment.
In a compelling review, researchers from Osaka Metropolitan University, along with their counterparts at Harvard Medical School, have delved into the intricate relationship between cell death mechanisms and cellular senescence, particularly their implications in cancer. The prevailing notion is that while senescent cells acquire resistance to traditional forms of programmed cell death, understanding the nuances of alternative inflammatory cell death pathways is crucial for therapeutic advancements. These alternative pathways include necroptosis, pyroptosis, and ferroptosis, which have recently gained recognition for their distinct molecular characteristics and potential roles in both health and disease.
The review emphasizes that, despite the recent advancements in understanding these cell death mechanisms, significant gaps remain, particularly concerning how senescent cells respond to these pathways. This knowledge is vital, as it may pave the way for novel therapeutic interventions aimed at either targeting senescent cells or mitigating their detrimental secretions without blatant removal, potentially preserving tissue homeostasis while alleviating cancer risk.
Moreover, the challenge lies not only in understanding these mechanisms but also in overcoming the inherent resistance that senescent cells have developed against apoptosis—the most well-characterized form of programmed cell death. Identifying strategies that can sensitize these resistant cells to apoptosis is of paramount importance for the efficacy of therapies designed to eliminate them. The review advocates for a multi-faceted approach in conducting further research to elucidate these cellular interactions and responses more comprehensively.
The researchers point out that as the landscape of cancer therapeutics evolves, an increasing focus on cellular senescence offers a promising avenue for innovation. Targeting senescent cells or the specific harmful factors they secrete can lead to a paradigm shift in cancer treatment—moving from conventional cytotoxic therapies towards more sophisticated, targeted strategies. These advances demonstrate the vital intersection between developmental biology, aging research, and cancer biology, revealing critical insights that could correlate to significant clinical outcomes.
Furthermore, the authors highlight the need for continued investigation into senomorphics—therapeutics that selectively target harmful aspects of the SASP without inducing cell death. This approach may provide a dual benefit: preserving beneficial aspects of senescence while mitigating its harmful influences. Such interventions could lead to improved patient outcomes, particularly in older individuals who are frequently more susceptible to cancer-related ailments owing to the accumulation of senescent cells.
In conclusion, the interconnectedness of cell death and senescence presents both challenges and opportunities for future research and therapeutic development. As knowledge expands, the potential to transform the current paradigms surrounding ageing and cancer treatment becomes increasingly attainable. Ultimately, the review aims to stimulate further exploration into these complex biological phenomena, hoping to lead to innovative strategies that can effectively tackle age-related diseases and cancer.
With the rapid pace of discovery in this field, the critical need for collaborative efforts between institutions will become more pronounced. Eliciting a robust dialogue amongst biologists, clinicians, and pharmaceutical researchers will ensure that the pathways leading to effective treatments are adequately explored. This collaborative approach could foster advancements that address the needs of an aging population, effectively combining knowledge from diverse spheres of research to yield beneficial outcomes for cancer therapies.
As stated by Dr. Kouhei Shimizu from Osaka Metropolitan University, the potential of understanding various forms of cell death in the context of cellular senescence is vast. It could significantly influence how we approach the development of next-generation cancer therapies. The hope is that as the mechanisms underlying these complex cellular changes are unravelled, researchers will be able to develop drugs that not only eliminate harmful senescent cells but also mitigate their pro-tumorigenic effects, contributing to healthier aging.
This transformative research is crucial not just for the scientific community but for society at large, where the implications of aging populations continue to challenge healthcare systems globally. With continued investment in this area, we might find ourselves on the brink of breakthroughs that could revolutionize how we understand and treat conditions associated with cellular aging and senescence, ultimately aiming for a healthier tomorrow.
Subject of Research: Cells
Article Title: The interplay between cell death and senescence in cancer
News Publication Date: 16-Nov-2024
Web References: 10.1016/j.semcancer.2024.11.001
References: Seminars in Cancer Biology
Image Credits: Osaka Metropolitan University
Keywords: Health and medicine, Cancer research, Cell apoptosis, Cellular senescence, Drug development, Drug therapy, Cell therapies, Molecular targets, Drug interactions, Pathophysiology, Molecular mechanisms, Cellular regulation, Regulatory mechanisms, Drug research, Cell growth, Tumor growth.
Tags: aging population and cancer researchalternative pathways of programmed cell deathand ferroptosisbiological processes of cellular agingcellular senescence and cancer connectionimplications of senescence in age-related diseasesmechanisms of cell death in agingpyroptosisresearch collaboration between Osaka and Harvard Medical Schoolrole of inflammation in cancer progressionsenescence-associated secretory phenotypetherapeutic implications of necroptosistumor-promoting environment and senescent cells