BENTLEY UNIVERSITY
Credit: Bentley University
BENTLEY UNIVERSITY
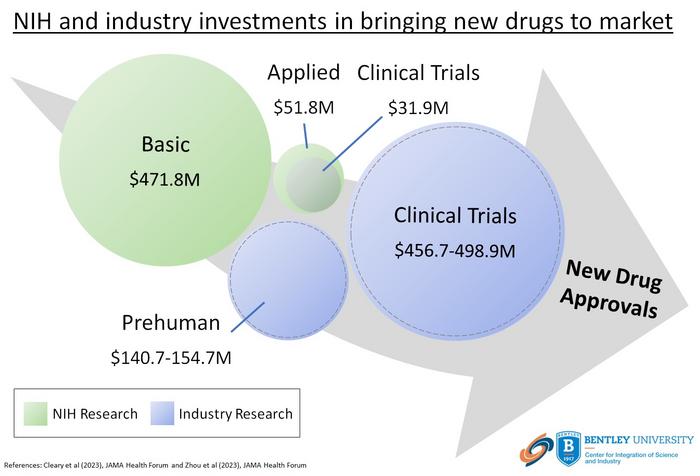
The U.S. National Institutes of Health (NIH) contributed $8.1 billion in project funding for phased clinical trials involving drugs approved by the FDA from 2010-2019, according to a new study from Bentley University’s Center for Integration of Science and Industry. The study, published in JAMA Health Forum, shows that NIH funding for clinical trials represents <3.5% of total NIH spending for basic or applied research related to these products and was significantly less than reported industry spending on clinical development.
The article, titled “Spending on phased clinical development of approved drugs by the US National Institutes of Health compared with industry“ is the first to broadly assess the NIH contribution to clinical development of new drugs and compare the scale of NIH spending relative to reported spending by industry. The study shows that NIH funding represented only ~10% of reported industry costs for phased clinical trials and was largely limited to programs designed to advance clinical and translational science in general by supporting centers, core capabilities, and training.
“This analysis confirms previous studies showing that the NIH makes substantive investments in the basic and applied science underlying new drugs, but also demonstrates that the NIH makes only limited contributions to development” said Fred Ledley, Director of the Center for Integration of Science and Industry, and the senior author on this study. “This is consistent with policies that position the public sector as an early investor in pharmaceutical innovations that are subsequently developed and commercialized by the pharmaceutical industry.”
This study identified $247 billion in total NIH funding contributing to more than 2.5 million publications describing basic or applied research related to 386 of 387 drugs approved 2010-2019 with $8.1 billion (3.3%) related to phased clinical development. This funding contributed to >12 thousand publications describing phased clinical trials involving 240 of 387 (62%) approved drugs. Average NIH spending was $33.8 million per approved drug and was significantly lower than reported industry spending. Overall, NIH spending represented ~10% of reported industry spending including ~25% of phase 1 costs, ~22% of phase 2 costs, and ~4% of phase 3 costs. More than 90% of NIH funding came through mechanisms designed to advance the practice of translational science or support programs or centers that provide clinical research capabilities, patient networks or consortia, or training in clinical, translational, or regulatory science (including Clinical Translational Science Awards). Only 3.3% of the total NIH funding was provided through mechanisms that support investigator-initiated research.
Dr. Edward Zhou was the lead author of this work along with Dr. Matthew Jackson and Dr. Ledley.
This work was supported by grants from the National Pharmaceutical Council and the National Biomedical Research Foundation.
THE CENTER FOR INTEGRATION OF SCIENCE AND INDUSTRY at Bentley University focuses on advancing the translation of scientific discoveries to create public value. The Center is an environment for interdisciplinary scholarship spanning basic science, data analytics, business, and public policy. For more information, visit www.bentley.edu/sciindustry and follow us on Twitter @sciindustry and LinkedIn.
BENTLEY UNIVERSITY is more than just one of the nation’s top business schools. It is a lifelong-learning community that creates successful leaders who make business a force for positive change. With a combination of business and the arts and sciences and a flexible, personalized approach to education, Bentley provides students with critical thinking and practical skills that prepare them to lead successful, rewarding careers. Founded in 1917, the university enrolls 4,100 undergraduate and 1,000 graduate and PhD students and is set on 163 acres in Waltham, Massachusetts, 10 miles west of Boston. For more information, visit bentley.edu. For more information, visit bentley.edu. Follow us on Twitter @BentleyU #BentleyUResearch.
Journal
JAMA Health Forum
DOI
10.1001/jamahealthforum.2023.1921
Method of Research
Observational study
Subject of Research
Not applicable
Article Title
Spending on Phased Clinical Development of Approved Drugs by the US National Institutes of Health Compared with Industry
Article Publication Date
14-Jul-2023
COI Statement
Drs Jackson and Ledley reported receiving grants to Bentley University from the Institute for New Economic Thinking and West Health Policy Center outside the submitted work. No other disclosures were reported.




