Credit: WFIRM
WINSTON-SALEM, NC, JULY 19, 2021 — Wake Forest researchers and clinicians are using patient-specific tumor ‘organoid’ models as a preclinical companion platform to better evaluate immunotherapy treatment for appendiceal cancer, one of the rarest cancers affecting only 1 in 100,000 people. Immunotherapies, also known as biologic therapies, activate the body’s own immune system to control, and eliminate cancer.
Appendiceal cancer is historically resistant to systemic chemotherapy, and the effect of immunotherapy is essentially unknown because clinical trials are difficult to perform due to lack of adequate patient numbers, resulting in a lack of data and limited research models.
Researchers at the Wake Forest Organoid Research Center (WFORCE), a joint venture between the Wake Forest Institute for Regenerative Medicine (WFIRM), and the Wake Forest Comprehensive Cancer Center, were the first to create appendiceal cancer organoids to use as a predictive model for potential treatment options (published 2018). The Comprehensive Cancer Center is a major high volume center with a global reputation in the treatment of appendiceal cancer.
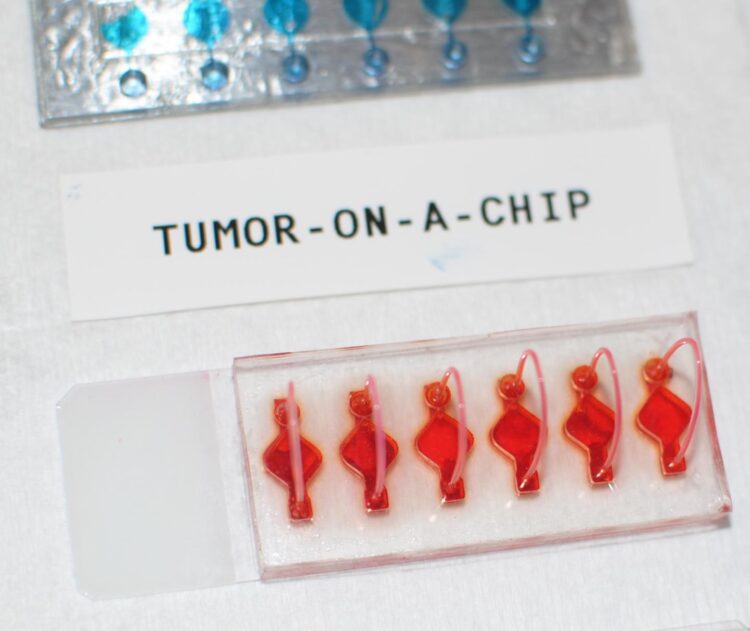
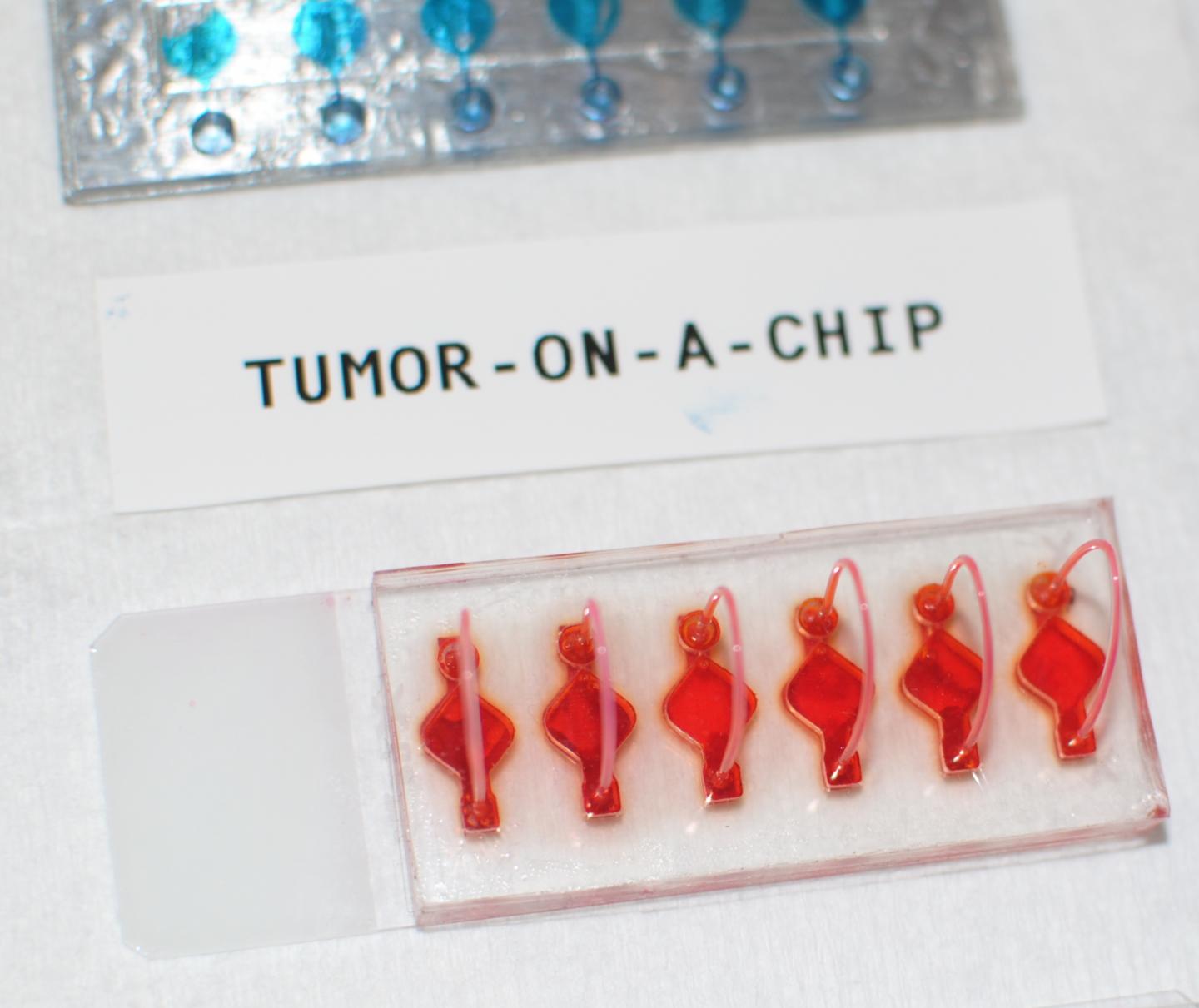
These cancer organoids are part of WFIRM’s “Body-on-a-Chip” system that allows scientists to engineer the organoids, or human tissue equivalents, that function in a very similar manner as actual human tissues and organs.
In this new study, published in the journal Clinical Cancer Research, their results indicate that various types immunotherapies tested on the organoids can potentially support treatment decisions and can achieve personalized results, identifying beneficial treatments while sparing patients from harmful side effects of drugs for which they will obtain no benefit.
“For this study we reconstructed patients’ tumors as organoids, supercharged with a built-in immune system directly obtained from the patient,” said senior author Konstantinos I. Votanopoulos, MD, PhD, professor of surgery at the Comprehensive Cancer Center and co-director of WFORCE. “In this way we created a personalized interface to study how effective the immunotherapy drugs are in activating a patient’s own immune system to kill the cancer. This platform is breaking new ground for appendiceal cancer, and it can also be applied in research for other rare cancers where preclinical models are lacking.”
This research study utilizes the WFIRM’s “Body-on-a-Chip” system that allows scientists to engineer the organoids, or humanoid tissue equivalents, that function in a very similar manner as actual human organs.
Cells from tumor biopsies from 26 patients were obtained to grow the organoids – tiny, 3D tissue-like structures, in the lab that that mimic the cancerous tumors. The immune enhanced tumor organoids were treated with one of three immunotherapy drugs and then assessed for responsiveness.
“In the future, by verifying that that the tumor and its organoids behave in the same fashion, we could modify clinical trial design and optimize cost by targeting patients with organoids that have exhibited favorable results,” Votanopoulos said.
Current strategies to understand tumor progression center on analyses of the tumor cells in isolation, but do not capture the interactions between a tumor and its surrounding space, known as the microenvironment or stroma. This leads to inaccuracies in predicting tumor progression and chemotherapy or immunotherapy response. Patient-derived tumor organoids are used as a testing and predicting platform to model diseases, evaluate efficacy and/or toxicity of new and existing drugs, and can be used to test environmental hazards.
Co-author Shay Soker, PhD, professor of regenerative medicine who leads tumor organoid research at WFIRM and co-directs WFORCE, said new technologies and biological models that improve prognostication will have a significant effect on patient mortality. “Using the organoids as a preclinical platform can lead to development of novel therapeutics which target and control tumor cells specifically, sparing healthy tissue from the side effects of chemotherapy and immunotherapy treatments,” he said. “For rare cancers like appendiceal cancer, this technology can make a difference in overall quality of life for patients.”
###
Additional co-authors include: Steven D Forsythe, MS, Richard A Erali, MD, Shyama Sasikumar, MS, Preston Laney, BS, Ethan Shelkey, BS, all of WFIRM; and Ralph D’Agostino Jr, PhD, Lance D Miller, PhD, Perry Shen, MD, and Edward A Levine, MD, all of the Comprehensive Cancer Center. The author’s report no conflicts of interest.
Research support provided by: the Wake Forest Dean’s Hero Award, the Appendix Cancer Pseudomyxoma Peritonei Foundation, the National Organization of Rare Diseases, and the National Institute of Health. Results were first presented at the Society of Surgical Oncology Annual Meeting, March 2021.
About the Wake Forest Institute for Regenerative Medicine: The Wake Forest Institute for Regenerative Medicine is recognized as an international leader in translating scientific discovery into clinical therapies, with many world firsts, including the development and implantation of the first engineered organ in a patient. Over 400 people at the institute, the largest in the world, work on more than 40 different tissues and organs. A number of the basic principles of tissue engineering and regenerative medicine were first developed at the institute. WFIRM researchers have successfully engineered replacement tissues and organs in all four categories – flat structures, tubular tissues, hollow organs and solid organs – and 15 different applications of cell/tissue therapy technologies, such as skin, urethras, cartilage, bladders, muscle, kidney, and vaginal organs, have been successfully used in human patients. The institute, which is part of Wake Forest School of Medicine, is located in the Innovation Quarter in downtown Winston-Salem, NC, and is driven by the urgent needs of patients. The institute is making a global difference in regenerative medicine through collaborations with over 400 entities and institutions worldwide, through its government, academic and industry partnerships, its start-up entities, and through major initiatives in breakthrough technologies, such as tissue engineering, cell therapies, diagnostics, drug discovery, biomanufacturing, nanotechnology, gene editing and 3D printing.
About Wake Forest Organoid Research Center: The Wake Forest Organoid Research Center (WFORCE) is a joint effort between the Wake Forest Institute for Regenerative Medicine and the Wake Forest Comprehensive Cancer Center and brings together researchers and clinicians, who are working side by side, to leverage the use of tissue organoid technology for the benefit of patients.
Media Contact
Bonnie Davis
[email protected]
Related Journal Article
http://dx.