All these preclinical data strongly suggest that the inhibition of mTORC1 and PLK1 proteins may be a promising therapeutic approach for NSCLC patients
Oncotarget published “High in vitro and in vivo synergistic activity between mTORC1 and PLK1 inhibition in adenocarcinoma NSCLC” which reported that the aim of this study was therefore to define combination of treatment based on the determination of predictive markers of resistance to the mTORC1 inhibitor RAD001/Everolimus.
When looking at biomarkers of resistance by RT-PCR study, three genes were found to be highly expressed in resistant tumors, i.e., PLK1, CXCR4, and AXL.
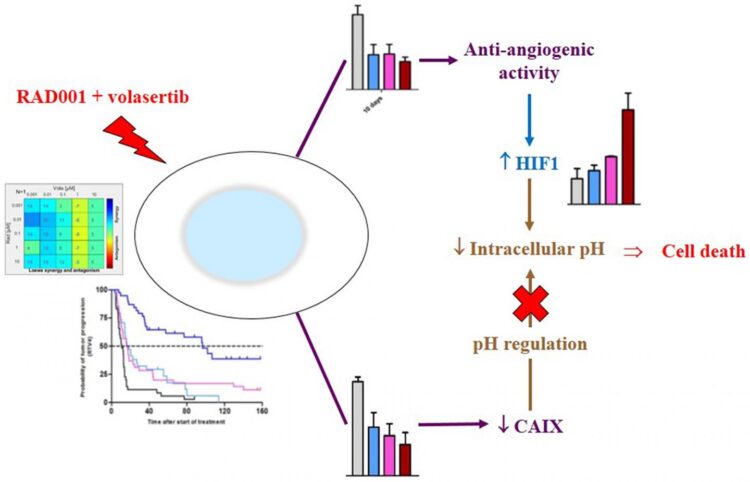
The authors have then focused this study on the combination of RAD001 Volasertib, a PLK1 inhibitor, and observed a high antitumor activity of the combination in comparison to each monotherapy; similarly, a clear synergistic effect between the two compounds was found in an in vitro study.
Pharmacodynamics study demonstrated that this synergy was due to tumor vascularization decrease, increase of the HIF1 protein expression and decrease of the intracellular pH, and decrease of the Carbonic Anhydrase 9 protein that could not correct intracellular acidosis.
In conclusion, all these preclinical data strongly suggest that the inhibition of mTORC1 and PLK1 proteins may be a promising therapeutic approach for NSCLC patients.
All these preclinical data strongly suggest that the inhibition of mTORC1 and PLK1 proteins may be a promising therapeutic approach for NSCLC patients
Dr. Didier Decaudin from The Institut Curie said, “Specifically targeting the Pi3K signaling pathway in the treatment of non-small cell lung cancers (NSCLC) has now been proposed for more than ten years.“
However, surprisingly, in the context of tumors defined by a Pi3K activation, very few clinical trials have tested single-agent Pi3K signaling pathway, all of them showing in fact a modest activity in molecularly unselected NSCLC patients, with the mTORC1 inhibitor everolimus, and the pan-Pi3K inhibitor Taselisib or Buparlisib.
Based on this rationale for the Pi3K pathway targeting in NSCLC, a huge number of clinical trials have consequently tested various Pi3K-based combination of treatments, including chemotherapies such as pemetrexed, taxanes, platinum salts, EGFR-TKi, radiotherapy, and others.
However, almost all of these combinations have been defined according to standard chemotherapies or NSCLC mutations, but not according to specific targets identified as biomarkers of resistance to Pi3K-targeting treatments.
The main strategy was therefore, using a panel of NSCLC PDXs, to define predictive markers of response to RAD001 therapy and to identify possible combinations of treatments that may be able to reverse RAD001 resistance.
Hence, using two well-documented Pi3K- and mTORC1-targeted therapies, i.e., BKM120 and RAD001, they have first evaluated in vivo responses in NSCLC Patient-Derived Xenografts, second defined marker of resistance to these two therapies that inhibition could pharmacologically be proposed, and finally evaluated a new identified and more relevant combination of treatments.
The Decaudin Research Team concluded in their Oncotarget Research Output that the determination of relevant Pi3K-based therapeutic combination was not supported, by the presence of actual molecular abnormalities, nor by physician therapeutic practices, but by the identification of predictive markers of resistance to Pi3K-based monotherapies.
The step-by-step methodology presented here highlights the useful of appropriate preclinical models of human cancers, such as PDXs; this methodology could be summarized, as follow:
- Assessment and validation of an antitumor activity of a treatment X
- Determination of predictive marker of resistance to the compound X
- Identification of a second treatment Y able to reverse the dismal impact of a previously defined predictive marker of resistance, and
- Validation of its clinical relevance in terms of its prognostic impact, assessment and validation of an antitumor activity of the combination of the treatments X Y, comprehensiveness, as far as possible, of the mechanisms of action of this new treatment combination, and, finally, clinical assessment in cancer patients.
This methodology may promote more relevant clinical trials and avoid non-efficient combinations, inacceptable toxicities, and expensive and time-consuming studies.
###
DOI – https:/
Full text – https:/
Correspondence to – Didier Decaudin – [email protected]
Keywords –
NSCLC,
Pi3K signalling pathway,
mTORC1,
RAD001 (everolimus),
PLK1
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https:/
SoundCloud – https:/
Facebook – https:/
Twitter – https:/
LinkedIn – https:/
Pinterest – https:/
Reddit – https:/
Oncotarget is published by Impact Journals, LLC please visit https:/
Media Contact
RYAN JAMES JESSUP
[email protected]
Original Source
https:/
Related Journal Article
http://dx.