Tuberculosis is one of the top ten causes of death worldwide, infecting about one-quarter of the world’s population. Although it is treatable, the rise of multidrug-resistant tuberculosis poses a major threat to global health security, and has been declared by the World Health Organization as a global health emergency. Reduced access to diagnosis and treatment during the COVID-19 pandemic is expected to dramatically increase the number of tuberculosis infections. This will set global efforts to tackle the disease back several years.
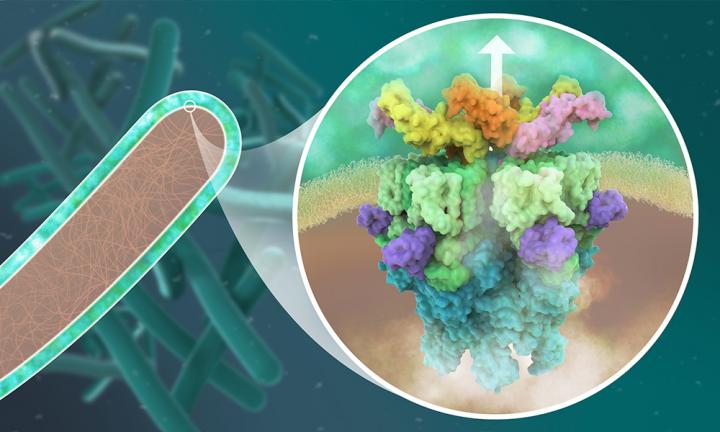
Tuberculosis is caused by infection with Mycobacterium tuberculosis: a bacterium that infects human lungs and other organs by using complex molecular machineries. These include protein complexes known as type VII secretion systems, which enable M. tuberculosis to release molecules into its host, which disarm and ultimately kill the infected human cell. Five such secretion systems, labelled ESX-1 to ESX-5, are found among M. tuberculosis and other closely related mycobacteria, many of which are pathogenic. Without them, the bacteria are unable to infect human cells.
The Wilmanns group at EMBL Hamburg has been using high-resolution structural biology to study mycobacterial proteins for the last two decades. The molecular understanding of the bacterial machinery used to infect cells resulted in collaborations with industry to develop new drugs against tuberculosis. In their most recent study, they determined the molecular structure of the secretion system ESX-5 to a high level of detail. They saw that the core of ESX-5 is built of 30 protein units, which form a dynamic membrane pore to allow secretion of proteins that enable the bacterium to survive and multiply inside human cells. Knowledge of the ESX-5 structure at high-resolution is essential to target specific sites with small-molecule drugs.
“Our new structure of the ESX-5 secretion complex provides deep insight into a major sluice gate that separates the inner of these bacteria from the outer host environment. Opening this gate allow the pathogen to spit out its deadly weapons to infect humans to develop tuberculosis. We can use this structure as a toolbox with literally thousands of potential drug targets. This will open an entirely new field of studies on tuberculosis,” says Matthias Wilmanns, who leads the study. Kate Beckham, who developed an innovative way to isolate ESX-5, adds: “The central pore we saw in ESX-5 could serve as a new drug target. Blocking it could prevent infection with pathogenic mycobacteria.”
The study could also help scientists to develop new vaccines for tuberculosis. The widely used Bacillus Calmette-Guérin (BCG) vaccine, which has its 100th anniversary this year, is based on a strain of mycobacterium that has lost its ability to cause disease because of a defect in the ESX-1 system. However, as BCG vaccination offers insufficient protection and is most effective in young infants only, so alternative vaccines are urgently needed. Due to its close structural and functional relation with ESX-1, targeting the ESX-5 secretion system might spur the development of new vaccines that could complement or replace those currently used.
Determining the molecular structure of ESX-5 was particularly challenging because of its large size and complexity. No single structural biology method can provide the full picture. In this case, the key to success was using integrative structural biology, in which data obtained using different methods -cryo-electron microscopy, X-ray crystallography, mass spectrometry and computational methods – were used jointly to create a coherent model.
“Eighteen months ago, solving this structure looked like mission: impossible,” says Matthias Wilmanns. “We managed to put the puzzle pieces together because each team member contributed unique expertise. To solve the complete structure, we collaborated with Jan Kosinski’s group at EMBL Hamburg and the Centre for Structural Systems Biology, which provided necessary expertise in integrative structural biology. We also received great help from our colleagues at EMBL Heidelberg, who performed cryo-electron microscopy experiments.”
This study illustrates some of EMBL’s approaches to life science research in its forthcoming scientific programme, Molecules to Ecosystems 2022-2026. As part of this programme, EMBL will take an interdisciplinary approach to understanding the molecular basis of life in the context of environmental changes. This will provide translational potential to support advances in human and planetary health.
EMBL’s approach, including this study, is aligned with the collaborative efforts of other research groups and institutions from Northern Germany working together at the Centre for Structural Systems Biology.
###
Media Contact
Lisa Vollmar
[email protected]
Original Source
https:/
Related Journal Article
http://dx.