Nanofiber device allows insulin to be released while defending against immune cells
A team of researchers led by diabetes specialists and biomedical engineers at Washington University School of Medicine in St. Louis and Cornell University has demonstrated that, using a miniscule device, they can implant insulin-secreting cells into diabetic mice. Once implanted, the cells secrete insulin in response to blood sugar, reversing diabetes without requiring drugs to suppress the immune system.
The findings are published June 2 in the journal Science Translational Medicine.
“We can take a person’s skin or fat cells, make them into stem cells and then grow those stem cells into insulin-secreting cells,” said Jeffrey R. Millman, PhD, an associate professor of medicine at Washington University and one of the study’s co-senior investigators. “The problem is that in people with Type 1 diabetes, the immune system attacks those insulin-secreting cells and destroys them. To deliver those cells as a therapy, we need devices to house cells that secrete insulin in response to blood sugar, while also protecting those cells from the immune response.”
In previous research, Millman, also an associate professor of biomedical engineering, developed and honed a method to make induced pluripotent stem cells, and to then grow those stem cells into insulin-secreting beta cells. Millman previously used those beta cells to reverse diabetes in mice, but it was not clear how the insulin-secreting cells might safely be implanted into people with diabetes.
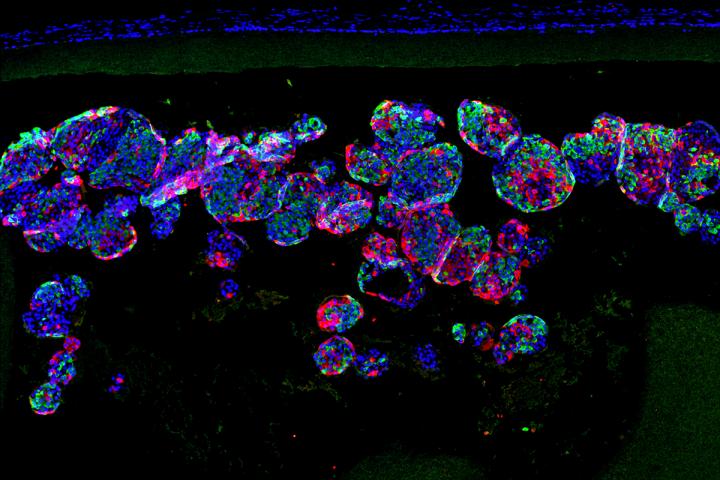
“The device, which is about the width of a few strands of hair, is micro-porous — with openings too small for other cells to squeeze into — so the insulin-secreting cells consequently can’t be destroyed by immune cells, which are larger than the openings,” said Millman. “One of challenges in this scenario is to protect the cells inside of the implant without starving them. They still need nutrients and oxygen from the blood to stay alive. With this device, we seem to have made something in what you might call a Goldilocks zone, where the cells could feel just right inside the device and remain healthy and functional, releasing insulin in response to blood sugar levels.”
Millman’s laboratory worked with researchers in the lab of Minglin Ma, PhD, an associate professor of biomedical engineering at Cornell. Ma has been working to develop biomaterials that can help implant beta cells safely into animals and, eventually, people with Type 1 diabetes.
Several implants have been tried in recent years, with varying levels of success. For this study, Ma, the study’s other co-senior investigator, and his colleagues developed what they call a nanofiber-integrated cell encapsulation (NICE) device. They filled the implants with insulin-secreting beta cells that had been manufactured from stem cells and then implanted the devices into the abdomens of mice with diabetes.
“The combined structural, mechanical and chemical properties of the device we used kept other cells in the mice from completely isolating the implant and, essentially, choking it off and making it ineffective,” Ma said. “The implants floated freely inside the animals, and when we removed them after about six months, the insulin-secreting cells inside the implants still were functioning. And importantly, it is a very robust and safe device.”
The cells in the implants continued to secrete insulin and control blood sugar in the mice for up to 200 days. And those cells continued to function despite the fact that the mice were not treated with anything to suppress their immune systems.
“We’d rather not have to suppress someone’s immune system with drugs, because that would make the patient vulnerable to infections,” Millman said. “The device we used in these experiments protected the implanted cells from the mice’s immune systems, and we believe similar devices could work the same way in people with insulin-dependent diabetes.”
Millman and Ma are reluctant to predict how long it might be before such a strategy could be employed clinically, but they plan to continue working toward that goal.
###
Wang X, et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Science Translational Medicine, published online June 2, 2021.
This work was supported by the Novo Nordisk Co., the Hartwell Foundation and by the Juvenile Diabetes Research Foundation. Additional funding from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH), grant numbers R01 DK105967, 5R01 DK114233 and T32 DK108742.
Washington University School of Medicine’s 1,500 faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is a leader in medical research, teaching and patient care, consistently ranking among the top medical schools in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
Media Contact
Jim Dryden
[email protected]
Original Source
https:/