Regeneration of muscle tissue was achieved by combining direct cell reprogramming with natural-synthetic hybrid scaffold as structural support
Credit: Institute for Basic Science
Muscle is the largest organ that accounts for 40% of body mass and plays an essential role in maintaining our lives. Muscle tissue is notable for its unique ability for spontaneous regeneration. However, in serious injuries such as those sustained in car accidents or tumor resection which results in a volumetric muscle loss (VML), the muscle’s ability to recover is greatly diminished. Currently, VML treatments comprise surgical interventions with autologous muscle flaps or grafts accompanied by physical therapy. However, surgical procedures often lead to a reduced muscular function, and in some cases result in a complete graft failure. Thus, there is a demand for additional therapeutic options to improve muscle loss recovery.
A promising strategy to improve the functional capacity of the damaged muscle is to induce de novo regeneration of skeletal muscle via the integration of transplanted cells. Diverse types of cells, including satellite cells (muscle stem cells), myoblasts, and mesenchymal stem cells, have been used to treat muscle loss. However, invasive muscle biopsies, poor cell availability, and limited long-term maintenance impede clinical translation, where millions to billions of mature cells may be needed to provide therapeutic benefits.
Another important issue is controlling the three-dimensional microenvironment at the injury site to ensure that the transplanted cells properly differentiate into muscle tissues with desirable structures. A variety of natural and synthetic biomaterials have been used to enhance the survival and maturation of transplanted cells while recruiting host cells for muscle regeneration. However, there are unsolved, long-lasting dilemmas in tissue scaffold development. Natural scaffolds exhibit high cell recognition and cell binding affinity, but often fail to provide mechanical robustness in large lesions or load-bearing tissues that require long-term mechanical support. In contrast, synthetic scaffolds provide a precisely engineered alternative with tunable mechanical and physical properties, as well as tailored structures and biochemical compositions, but are often hampered by lack of cell recruitment and poor integration with host tissue.
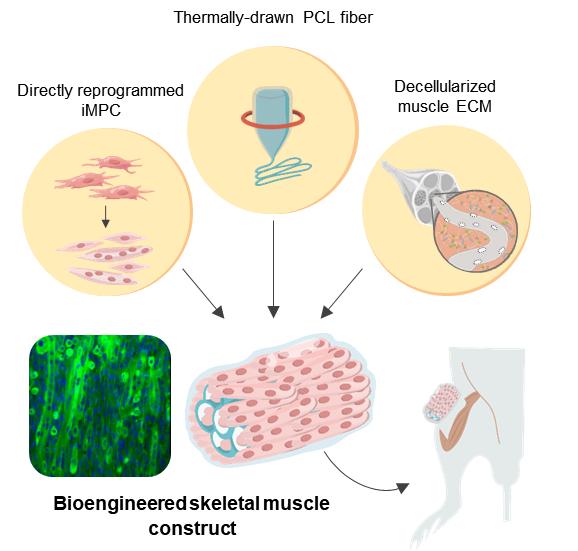
To overcome these challenges, a research team at the Center for Nanomedicine within the Institute for Basic Science (IBS) in Seoul, South Korea, Yonsei University, and the Massachusetts Institute of Technology (MIT) devised a novel protocol for artificial muscle regeneration. The team achieved effective treatment of VML in a mouse model by employing direct cell reprogramming technology in combination with a natural-synthetic hybrid scaffold.
Direct cell reprogramming, also called direct conversion, is an efficient strategy that provides effective cell therapy because it allows the rapid generation of patient-specific target cells using autologous cells from the tissue biopsy. Fibroblasts are the cells that are commonly found within the connective tissues, and they are extensively involved in wound healing. As the fibroblasts are not terminally differentiated cells, it is possible to turn them into induced myogenic progenitor cells (iMPCs) using several different transcription factors. Herein, this strategy was applied to provide iMPC for muscle tissue engineering.
In order to provide structural support for the proliferating muscle cells, polycaprolactone (PCL), was chosen as a material for the fabrication of a porous scaffold due to its high biocompatibility. While salt-leaching is a widely used method to create porous materials, it is mostly limited to producing closed porous structures. To overcome this limitation, the researchers augmented the conventional salt leaching method with thermal drawing to produce customized PCL fiber scaffolds. This technique facilitated high-throughput fabrication of porous fibers with controlled stiffness, porosity, and dimensions that enable precise tailoring of the scaffolds to the injury sites.
However, the synthetic PCL fiber scaffolds alone do not provide optimal biochemical and local mechanical cues that mimic muscle-specific microenvironment. Hence the construction of a hybrid scaffold was completed through the incorporation of decellularized muscle extracellular matrix (MEM) hydrogel into the PCL structure. Currently, MEM is one of the most widely used natural biomaterials for the treatment of VML in clinical practice. Thus, the researchers believe that hybrid scaffolds engineered with MEM have a huge potential in clinical applications.
The resultant bioengineered muscle fiber constructs showed mechanical stiffness similar to that of muscle tissues and exhibited enhanced muscle differentiation and elongated muscle alignment in vitro. Furthermore, implantation of bioengineered muscle constructs in the VML mouse model not only promoted muscle regeneration with increased innervation and angiogenesis but also facilitated the functional recovery of damaged muscles. The research team notes: “The hybrid muscle construct might have guided the responses of exogenously added reprogrammed muscle cells and infiltrating host cell populations to enhance functional muscle regeneration by orchestrating differentiation, paracrine effect, and constructive tissue remodeling.”
Prof. CHO Seung-Woo from the IBS Center for Nanomedicine and Yonsei University College of Life Science and Biotechnology who led this study notes: “Further studies are required to elucidate the mechanisms of muscle regeneration by our hybrid constructs and to empower the clinical translation of cell-instructive delivery platforms.”
###
Media Contact
William I. Suh
[email protected]




