A potential vaccine protects hamsters and mice against death caused by multiple Leptospira species, and prevents kidney colonization in mice
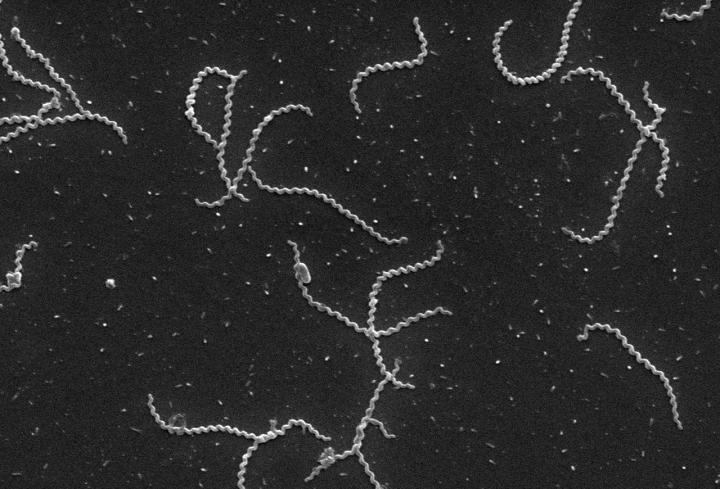
Credit: Wunder et al. (CC BY 4.0)
Scientists have designed a single-dose universal vaccine that could protect against the many forms of leptospirosis bacteria, according to a study published today in eLife.
An effective vaccine would help prevent the life-threatening conditions caused by leptospirosis, such as Weil’s disease and lung haemorrhage, which are fatal in 10% and 50% of cases, respectively.
Leptospirosis is caused by a diverse group of spirochetes called leptospires. A broad range of mammals, including rats, harbour the bacteria in their kidneys and release them into the environment through their urine. Humans and animals can then get infected after coming into contact with contaminated water or soil. In addition to having a major impact on the health of vulnerable human populations, leptospirosis is an economically important animal health problem, making it a significant One Health challenge. This means that collaborative efforts are needed across disciplines and sectors to improve public health outcomes against leptospirosis infection.
The Leptospira family of bacteria is made up of 64 species with 300 different varieties (called serovars). This makes developing a vaccine challenging, because researchers need to find a common feature of the bacteria that will trigger an immune response.
“We have recently identified a novel protein called FcpA in the flagella of Leptospira which enables it to move and penetrate human and animal tissues,” explains first author Elsio Wunder Jr, Associate Research Scientist in Epidemiology (Microbial Diseases) at Yale School of Public Health, Yale University, New Haven, US. “With this study, we wanted to see whether using engineered Leptospira that lacks a functional FcpA molecule has the potential for a vaccine that could provide major public health benefit.”
The mutated FcpA Leptospira was tested as an attenuated vaccine – a live vaccine that cannot cause disease. After the vaccine was given to hamsters and mice, it disseminated throughout the body before being cleared within seven days in the hamsters and after two weeks in the mice. No traces of the mutated Leptospira could be detected in kidney tissue or blood after this time point, showing that the attenuated vaccine is cleared by the immune system before it results in disease or death.
To test whether the vaccine candidate could protect against all types of Leptospira infection, they tested a single dose of the mutant Leptospira and compared this against heat-killed Leptospira to see whether they could prevent infection and disease by a range of similar and different serovars. Immunisation with the heat-killed vaccine gave partial protection against similar serovars but not against different serovars of Leptospira. By contrast, the attenuated vaccine (mutated Leptospira) provided cross-protection against serovars belonging to three different species of Leptospira, which encompass the majority of serovars of importance to human and animal health.
Further analysis of the mice and hamsters after vaccination showed that they generated antibodies that recognised a wide range of proteins across the different species of Leptospira. Moreover, by studying the antibody response in detail, the team identified 41 different proteins that could be targets for future vaccines. The majority of these proteins (70%) looked similar across all 13 disease-causing species of Leptospira studied, suggesting they are likely to be important to the microbes’ survival and would make effective future vaccine candidates.
“In this proof-of-concept study, we have shown that a universal leptospirosis vaccine candidate can prevent both death and kidney colonisation in animal models,” concludes author Albert Ko, Department Chair and Professor of Epidemiology (Microbial Diseases) at Yale School of Public Health. “These findings take us one step closer to achieving the holy grail for the field, which is an effective vaccine that protects against the many Leptospira species and can be deployed as a broad solution to the human and animal health challenge caused by leptospirosis.”
###
Reference
The paper ‘A live attenuated vaccine model confers cross-protective immunity against different species of the Leptospira genus’ can be freely accessed online at https:/
Media contacts
Emily Packer, Media Relations Manager
eLife
[email protected]
+44 1223 855373
Michael Greenwood, Senior Communications Officer
Yale School of Public Health
[email protected]
+1 (203) 737-5151
About eLife
eLife is a non-profit organisation created by funders and led by researchers. Our mission is to accelerate discovery by operating a platform for research communication that encourages and recognises the most responsible behaviours. We aim to publish work of the highest standards and importance in all areas of biology and medicine, including Microbiology and Infectious Disease, while exploring creative new ways to improve how research is assessed and published. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, the Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https:/
To read the latest Microbiology and Infectious Disease research published in eLife, visit https:/
Media Contact
Emily Packer
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




