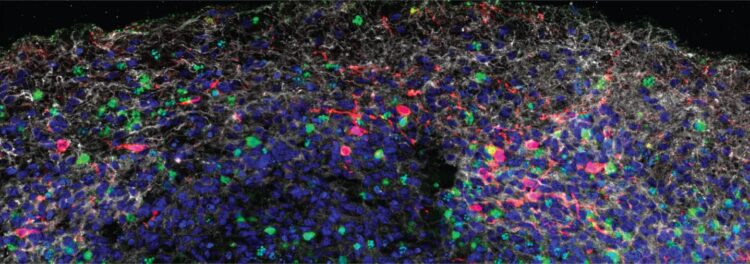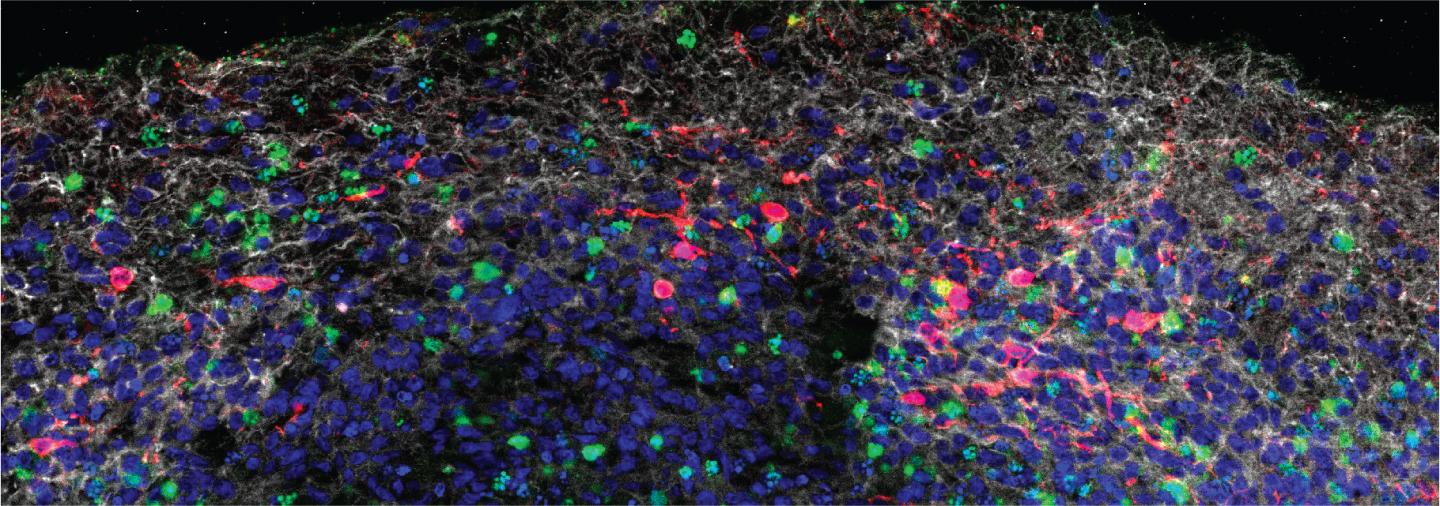
Credit: © 2021 Song et al. Originally published in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20202135
Using both mouse and human brain tissue, researchers at Yale School of Medicine have discovered that SARS-CoV-2 can directly infect the central nervous system and have begun to unravel some of the virus’s effects on brain cells. The study, published today in the Journal of Experimental Medicine (JEM), may help researchers develop treatments for the various neurological symptoms associated with COVID-19.
Though COVID-19 is considered to primarily be a respiratory disease, SARS-CoV-2 can affect many other organs in the body, including, in some patients, the central nervous system, where infection is associated with a variety of symptoms ranging from headaches and loss of taste and smell to impaired consciousness, delirium, strokes, and cerebral hemorrhage.
“Understanding the full extent of viral invasion is crucial to treating patients, as we begin to try to figure out the long-term consequences of COVID-19, many of which are predicted to involve the central nervous system,” says Akiko Iwasaki, a professor at Yale School of Medicine.
Many questions remain to be answered, including whether SARS-CoV-2 can infect neurons or other types of brain cells. To address this question, a team led by Iwasaki and co-senior author Kaya Bilguvar, an associate professor at Yale School of Medicine, analyzed the ability of SARS-CoV-2 to invade human brain organoids, miniature 3D organs grown in the lab from human stem cells. The researchers found that the virus was able to infect neurons in these organoids and use the neuronal cell machinery to replicate. The virus appears to facilitate its replication by boosting the metabolism of infected cells, while neighboring, uninfected neurons die as their oxygen supply is reduced.
SARS-CoV-2 enters lung cells by binding to a protein called ACE2, but whether this protein is present on the surface of brain cells is unclear. The Yale team determined that the ACE2 protein is, in fact, produced by neurons and that blocking this protein prevents the virus from human brain organoids.
SARS-CoV-2 was also able to infect the brains of mice genetically engineered to produce human ACE2, causing dramatic alterations in the brain’s blood vessels that could potentially disrupt the organ’s oxygen supply. Central nervous system infection was much more lethal in mice than infections limited to the lungs, the researchers found.
Finally, the researchers analyzed the brains of three patients who succumbed to COVID-19. SARS-CoV-2 was detected in the cortical neurons of one of these patients, and the infected brain regions were associated with ischemic infarcts in which decreased blood supply causes localized tissue damage and cell death. Microinfarcts were detected in the brain autopsy of all three patients.
“Our study clearly demonstrates that neurons can become a target of SARS-CoV-2 infection, with devastating consequences of localized ischemia in the brain and cell death,” Bilguvar says. “Our results suggest that neurologic symptoms associated with COVID-19 may be related to these consequences, and may help guide rational approaches to the treatment of COVID-19 patients with neuronal disorders.”
“Future studies will be needed to investigate what might predispose some patients to infections of the central nervous system and to determine the route of SARS-CoV-2 invasion into the brain and the sequence of infection in different cell types within the central nervous system that will help validate the temporal relationship between SARS-CoV-2 and ischemic infarcts in patients,” Iwasaki adds.
###
Song et al., 2021. J. Exp. Med. https:/
About the Journal of Experimental Medicine
The Journal of Experimental Medicine (JEM) features peer-reviewed research on immunology, cancer biology, stem cell biology, microbial pathogenesis, vascular biology, and neurobiology. All editorial decisions are made by research-active scientists in conjunction with in-house scientific editors. JEM makes all of its content free online no later than six months after publication. Established in 1896, JEM is published by the Rockefeller University Press. For more information, visit jem.org.
Visit our Newsroom, and sign up for a weekly preview of articles to be published. Embargoed media alerts are for journalists only.
Follow JEM on Twitter at @JExpMed and @RockUPress.
Media Contact
Ben Short
[email protected]
Original Source
https:/
Related Journal Article
http://dx.