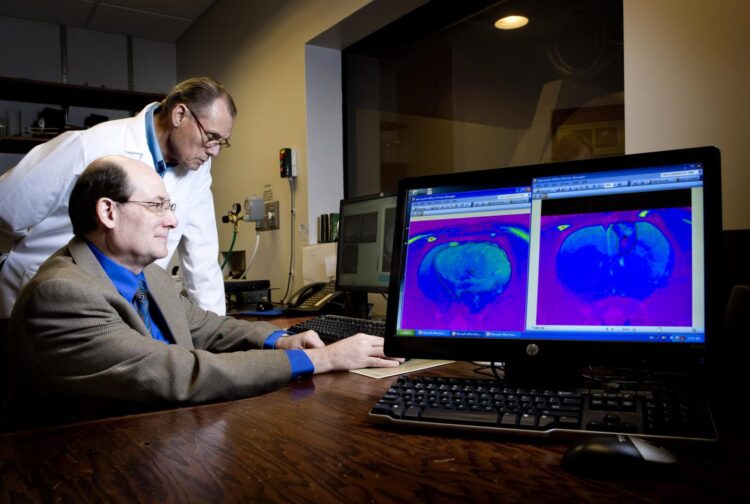
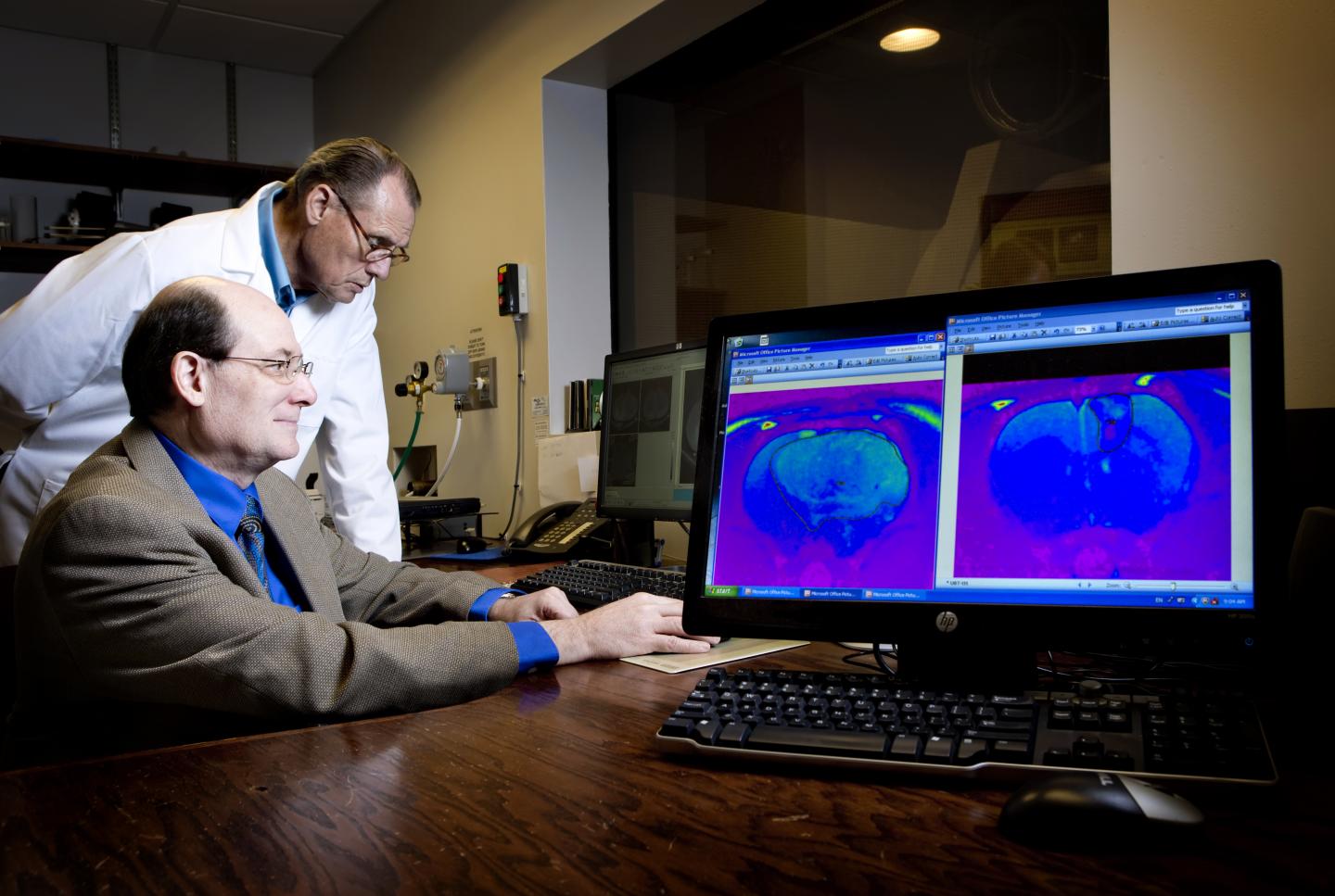
The drug treats fast-growing, deadly brain cancers with no effective treatment
Credit: Oklahoma Medical Research Foundation
The U.S. Food and Drug Administration has awarded Rare Pediatric Disease Designation (RPDD) for diffuse intrinsic pontine glioma (DIPG) and Orphan Drug Designation for treatment of malignant glioma to OKN-007, an investigational drug discovered at the Oklahoma Medical Research Foundation and being developed by Oblato, Inc.
DIPG is a fast-growing pediatric cancer that starts in the brain stem. It is one of several sub-categories of malignant gliomas, deadly cancers of the brain and spinal cord.
“We are very pleased to receive the successful designations from the FDA for our proprietary compound OKN-007,” said Oblato President and Chief Executive Officer Won S. Yang. These designation programs provide for special status and priority review of regulatory applications for new therapies for rare pediatric or “orphan” diseases, conditions that affect limited patient populations.
According to the National Brain Tumor Society, approximately 26,000 Americans will be diagnosed with primary malignant brain tumors this year. Of those, DIPG.org reports that up to 300 will be cases of DIPG.
OKN-007 was initially discovered by OMRF scientists Rheal Towner, Ph.D., and Robert Floyd, Ph.D. Oblato acquired all rights to OKN-007 from OMRF, and the company is currently testing the investigational drug in a Phase 2 clinical study of 56 patients suffering from recurrent glioblastoma, the most aggressive form of glioma. The patients are being treated with the drug in combination with another medication, temozolomide, at eight sites in the U.S.
In pre-clinical studies at OMRF, Towner has also shown that OKN-007 inhibits growth of human DIPG tumors implanted in experimental models. Oblato is planning to begin clinical trials in DIPG patients in 2021. “Right now, there is no effective treatment for this deadly brain cancer,” said Towner.
Going forward, Oblato and OMRF will continue collaborating, with a focus on improving treatment for patients suffering from a variety of solid-tumor cancers.
“OMRF and Oblato are committed to a single goal: helping patients overcome these life-threatening illnesses,” said OMRF Director of Technology Ventures Andrew Westmuckett, Ph.D. “Our hope is that OKN-007 can transform the therapeutic landscape.”
###
About Oblato
Oblato, a wholly-owned subsidiary of Korean biotech company GtreeBNT, is incorporated in Delaware and has its principal place of business in New Jersey.
About OMRF
OMRF (omrf.org) is an independent, nonprofit biomedical research institute dedicated to understanding and developing more effective treatments for human diseases.
Media Contact
Ryan Stewart
[email protected]