Scientists at EPFL’s Brain Mind Institute have identified an enzyme that can play a central role in developing a new route of treatment for Huntington’s Disease.
Credit: R.N. Hegde and H. Lashuel (EPFL)
Huntington disease is a progressive and aggressively debilitating brain disorder that causes uncontrolled movements, psychological problems, and loss of cognition. It is caused by a mutation in the gene that encodes the protein huntingtin, causing it to build an abnormally long tail of the amino acid glutamine. This tail prevents huntingtin from folding properly and as a result it aggregates inside neurons of the brain, and eventually kills them.
Huntington’s affects hundreds of thousands of people in the world, and as an “autosomal dominant” disease, a person only needs one copy of the mutant huntingtin gene to develop the disease. Scientists in both academia and industry are exploring different approaches to tackle the disease. The most popular strategy is to lower the levels of huntingtin or to inhibit its aggregation – or a combination of both. The way to do this is to either “silence” the huntingtin gene or to activate cellular mechanisms that promote the degradation of the protein itself.
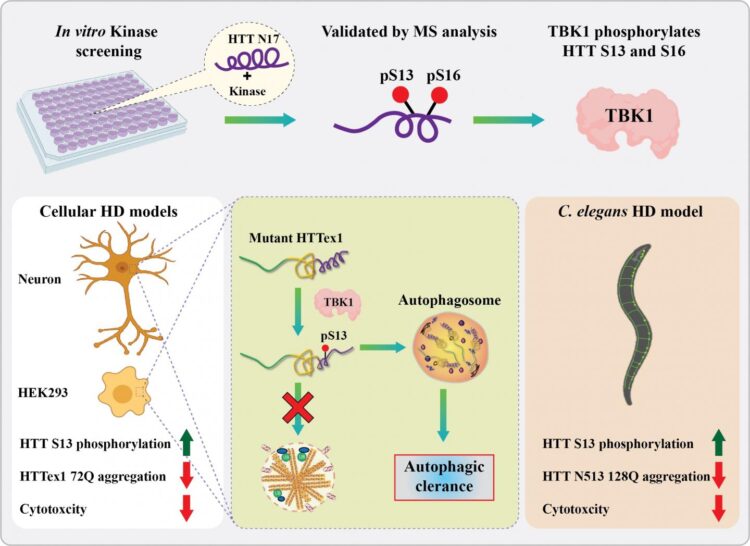
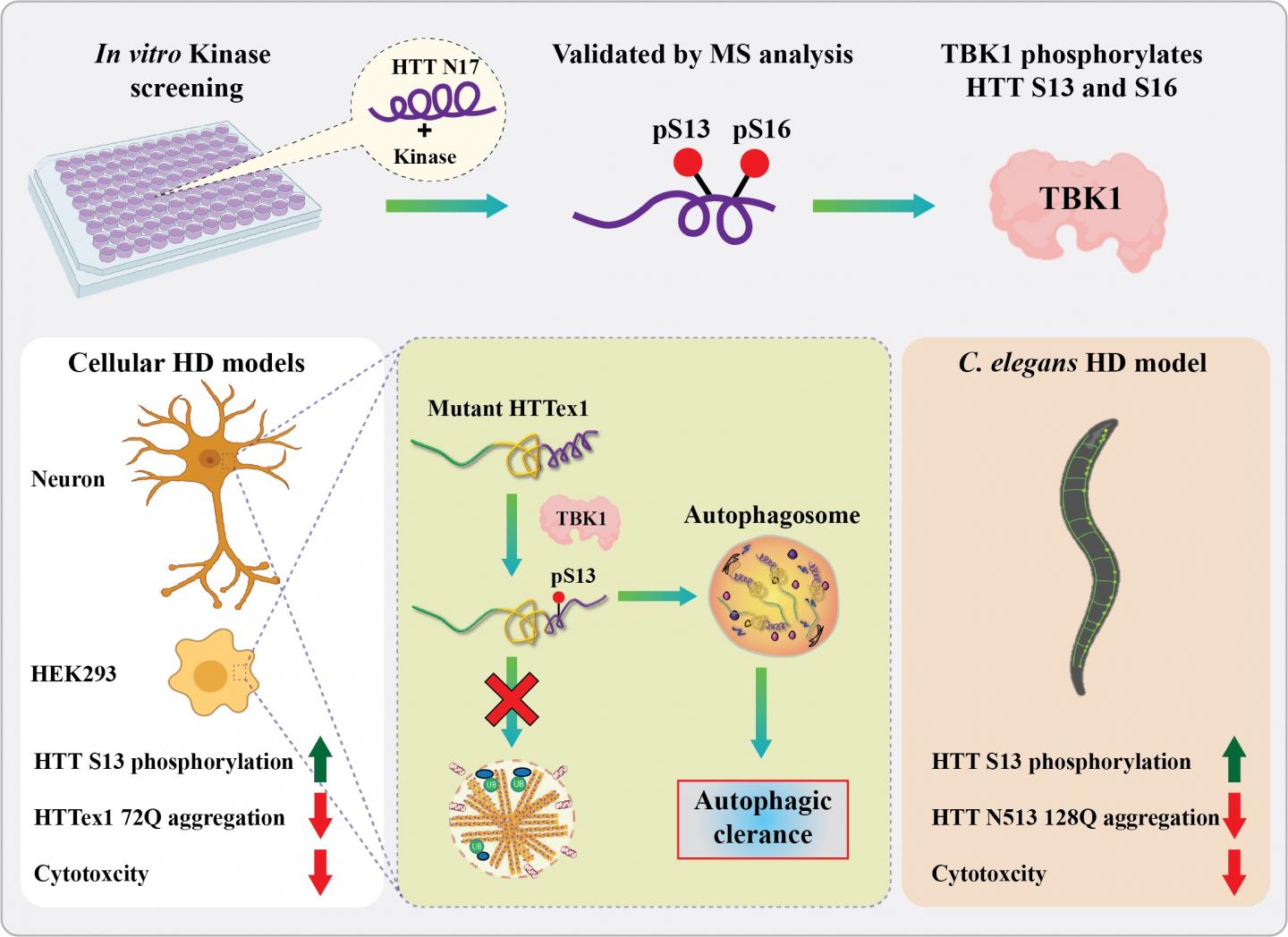
Now, scientists at the lab of Professor Hilal Lashuel at EPFL have identified a new enzyme that does both. The enzyme, called “TBK1”, plays a central role in regulating the degradation and clearance of the huntingtin protein and introduces chemical modifications that block its aggregation. “We believe that this represents a viable target for the development of possible treatment of Huntington’s disease,” says Lashuel.
The TBK1 enzyme is a “kinase”. In the cell, kinases are enzymes that add phosphate groups to various biomolecules like proteins or DNA. In the world of the cell, phosphate groups are energy-carriers, so adding one essentially “turns on” the receiving molecule.
Previous studies have shown that artificially adding phosphate groups to huntingtin can stop it from aggregating and causing Huntington’s disease. “However, to explore the therapeutic potential of phosphorylation, we needed to identify the natural kinases that do the job inside the cell,” says Lashuel. “After screening hundreds of kinases, we were excited to identify TBK1, because it did the job with high specificity and efficiency.”
The researchers found that, when TBK1 adds a phosphate group anywhere in the first 17 amino acids of huntingtin, it inhibits its ability to aggregate. This was the case for both the normal and mutated versions of huntingtin.
In addition, increasing TBK1 levels in cells leads to over-phosphorylation of a specific amino acid (a serine) in the huntingtin chain. This stabilizes the protein and stops it from aggregating.
Finally, TBK1 was also found to signal the cell to degrade and clean out huntingtin before it aggregates. This lowers overall huntingtin levels, which results in reducing aggregate formation inside the cell.
Encouraged by their findings, the scientists then moved onto an animal model of Huntington’s Disease: the worm C. elegans. What they found corroborated their previous data: Over-expressing the TBK1 kinase protected against mutant huntingtin toxicity in the worm, preventing the development of Huntington’s Disease. The researchers got similar results in cultured neurons.
“Our work shows that TBK1-mediated increase in phosphorylation and/or promoting mutant huntingtin autophagic clearance represent viable therapeutic strategies for the treatment of Huntington’s Disease,” says Ramanath Hegde, who led the study.
“We are very excited about these findings,” says Lashuel. “TBK1 has also been shown to regulate the clearance and degradation of proteins implicated in other neurodegenerative diseases. Mutations in TBK1 have also recently been linked to ALS and result in impaired autophagy, which leads to the accumulation of aggregates. Our goal is to find small molecules or drug pathways and to develop these for multiple neurodegenerative diseases.”
###
Other contributors
IRBM Science Park, Italy
Johns Hopkins University School of Medicine
EPFL Laboratory of Integrative and Systems Physiology
University College London
University of Auckland
Media Contact
Hilal Lashuel
[email protected]
Original Source
https:/
Related Journal Article
http://dx.