Researchers find fast rates of morphological evolution do not need to coincide with taxonomic diversification as predicted by the classical theory of adaptive radiation
Credit: Courtesy Tiago R. Simões
Some of the most fundamental questions in evolution remain unanswered, such as when and how extremely diverse groups of animals – for example reptiles – first evolved. For seventy-five years, adaptive radiations – the relatively fast evolution of many species from a single common ancestor – have been considered as the major cause of biological diversity, including the origins of major body plans (structural and developmental characteristics that identify a group of animals) and new lineages. However, past research examining these rapid rates of evolution was largely constrained by the methods used and the amount of data available.
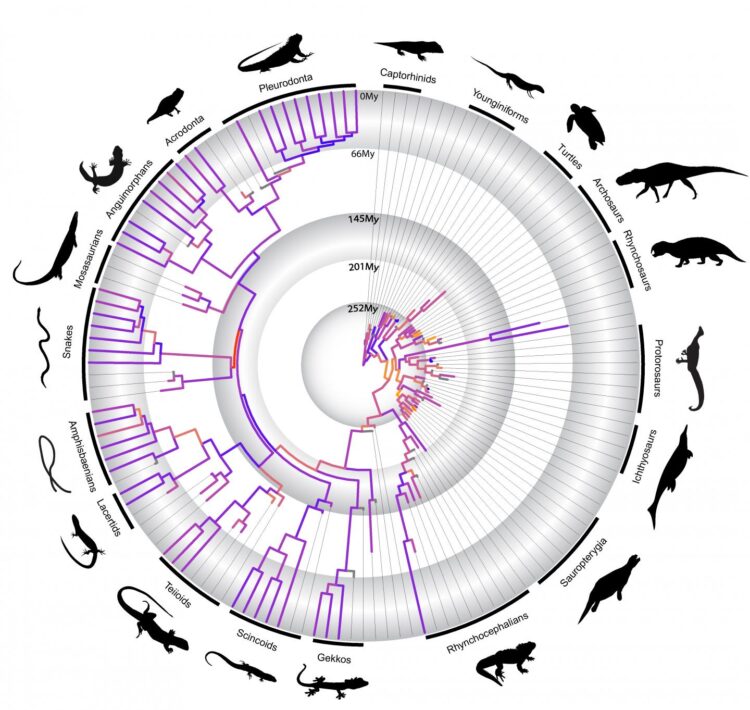
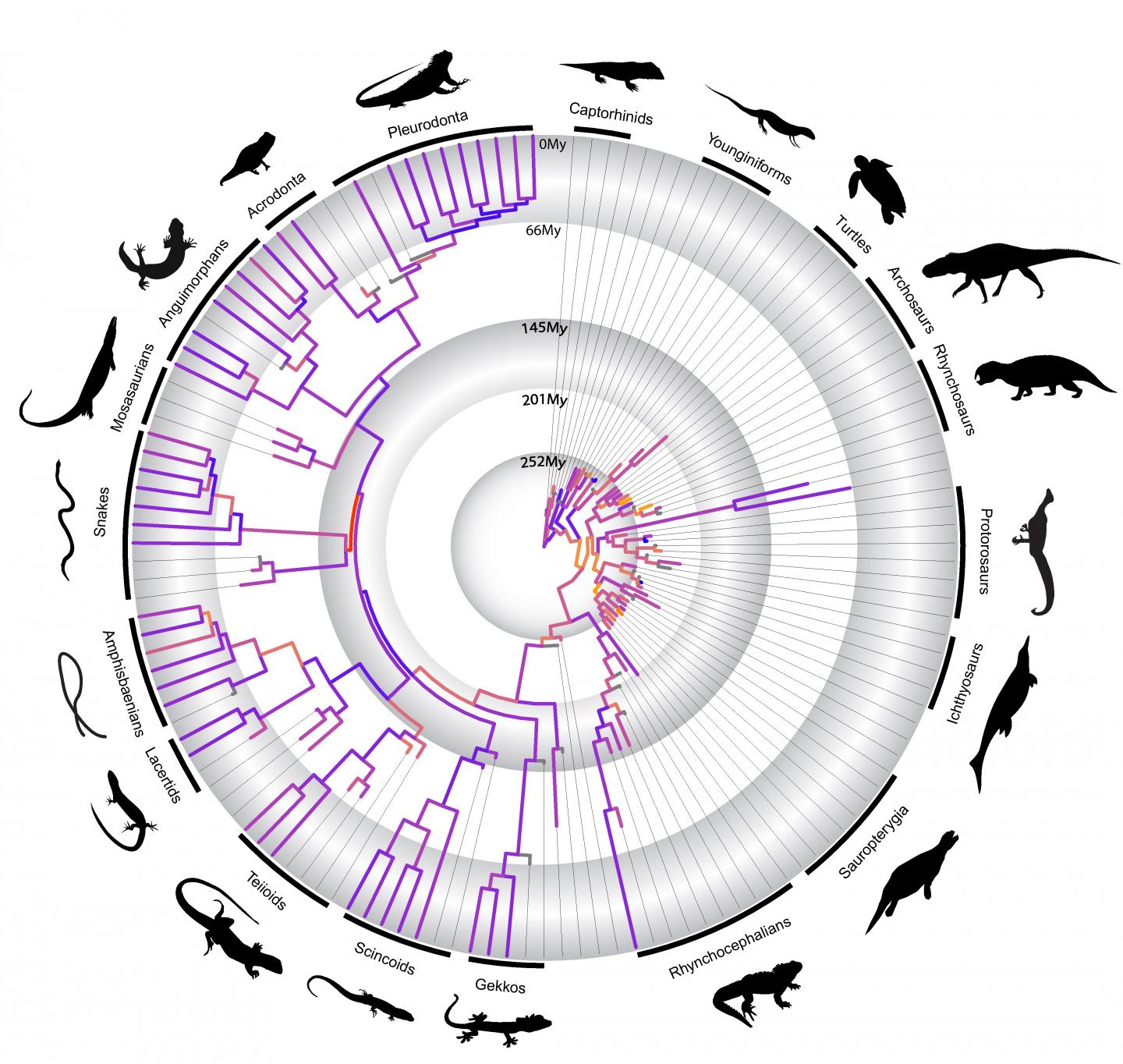
In a paper out today in Nature Communications, a research team lead by Harvard University examined the largest available data set of living and extinct major reptile groups (such as marine reptiles, turtles, lizards, and the ancestors of dinosaurs and crocodiles) to tackle the longstanding question of how adaptive radiations have shaped reptile evolution. Using DNA information from modern species and hundreds of anatomical features from both modern and fossil species for statistical analysis, the study detected that periods of fast anatomical change during the origin of reptile groups often predate when those groups diversified into hundreds or thousands of species. This contradicts long-held ideas of adaptive radiation in evolution biology.
“Our findings suggest that the origin of the major reptile groups, both living and extinct, was marked by very fast rates of anatomical change, but that high rates of evolution do not necessarily align with taxonomic diversification” said first author Dr. Tiago Simões, Postdoctoral Fellow in in the lab of Stephanie Pierce, Associate
Professor in the Department of Organismic and Evolutionary Biology at Harvard University.
Simões and Pierce revealed that rates of evolution and morphological variety in reptiles prior to the Permian-Triassic Mass Extinction – the biggest mass extinction of all time – were equally high, or even higher, than after the event. As reptile species diversity was much lower during the Permian compared to Triassic, these results indicate that fast rates of evolution do not need to coincide with rapid taxonomic diversification as predicted by the classical theory of adaptive radiation. The two can be decoupled.
The team, which also included PhD student Oksana Vernygora and Professor Michael Caldwell at the University of Alberta, further discovered that accelerated rates of evolution correspond to the origin of unique reptile body plans, but that very similar functional adaptations in reptiles can arise through varying rates of evolution.
“Surprisingly,” Pierce said, “reptiles that evolved similar protective armour like turtles or serpentine bodies like snakes, show radically different rates of evolution, indicating the origin and evolution of unique body plans is heterogeneous through evolution.”
“Our results also show that the origin of snakes is characterized by the fastest rates of anatomical change in the history of reptile evolution,” said Simões. “But, that this does not coincide with increases in taxonomic diversity [as predicted by adaptive radiations] or high rates of molecular evolution.”
The mismatch between morphological and molecular evolution supports the idea that protein coding DNA sequences do not seem to be correlated with broad-scale changes in anatomy. Although much more research is needed to understand how body plans evolve, the team hypothesizes that non-protein coding regions of the genome may be responsible for rapid morphological change, as these parts are more free to mutate and take on new functional roles.
“It is clear to us that to advance our understanding of the major patterns in evolution we need further studies capable of measuring phenotypic and molecular evolutionary rates, times of origin, and phenotypic diversity across large timescales” said Simões.
Simões and colleagues continue to develop new methods and are expanding their data set back in time to look at the origins of amniotes, the group that includes both reptiles
and mammals. Of particular interest is pinpointing when in geological time these two groups of animals diverged and how extinction, diversification, and adaptation have shaped their evolutionary history over the last 300+ million years.
“I’m excited to continue my research to unravel the early evolutionary dynamics of the two most successful groups of animals on the planet,” Simões said. “I’m also focusing on improving available protocols to analyze morphological data and construct more robust evolutionary trees, including the timing of origin of major vertebrate lineages.”
###
Article and author details
Tiago R. Simões, Oksana Vernygora, Michael W. Caldwell and Stephanie E. Pierce. 2020. Megaevolutionary dynamics and the timing of evolutionary innovation in reptiles. Nature Communications 11.
doi: 10.1038/s41467-020-17190-9
Corresponding authors
Tiago R. Simões, [email protected]
Media Contact
Wendy Heywood
[email protected]
Related Journal Article
http://dx.