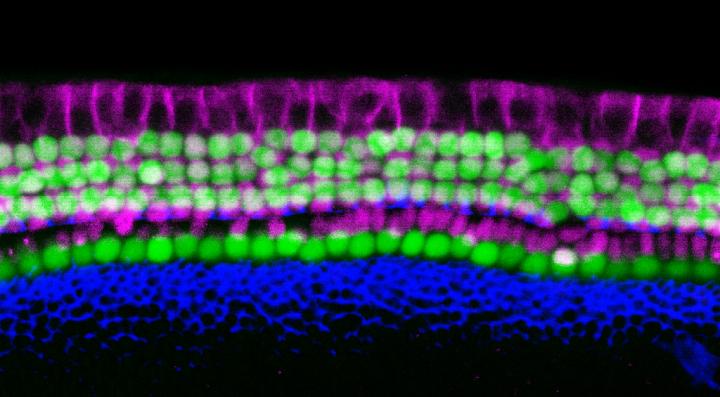
Credit: Image by Yassan Abdolazimi/Segil Lab/USC Stem Cell
Scientists from the USC Stem Cell laboratories of Neil Segil and Justin Ichida are whispering the secrets of a simpler way to generate the sensory cells of the inner ear. Their approach uses direct reprogramming to produce sensory cells known as “hair cells,” due to their hair-like protrusions that sense sound waves. The study was published in the journal eLife.
“We’ve succeeded in directly reprogramming a variety of mouse cell types into what we’re calling ‘induced hair cell-like cells, or iHCs,” said PhD student Louise Menendez, the study’s lead author. “This allows us to efficiently generate large numbers of iHCs to identify causes and treatments for hearing loss.”
The scientists successfully reprogrammed three different types of mouse cells to become iHCs. The first two types were embryonic and adult versions of connective tissue cells, known as fibroblasts. The third was a different type of inner ear cell, known as a supporting cell.
To achieve reprogramming, the scientists exposed fibroblasts and supporting cells to a cocktail of four transcription factors, which are molecules that help convey the instructions encoded in DNA. The scientists identified this cocktail by testing various combinations of 16 transcription factors that were highly active in the hair cells of newborn mice.
“The four key ingredients turned out to be the transcription factors Six1, Atoh1, Pou4f3, and Gfi1,” said Menendez.
The resulting iHCs resembled naturally occurring hair cells in terms of their structure, electrophysiology, and genetic activity. The iHCs also possessed several other distinct characteristics of hair cells, including vulnerability to an antibiotic known to cause hearing loss.
“Hair cells are easy to damage, and currently impossible to repair in humans,” said Segil, a professor in the Department of Stem Cell Biology and Regenerative Medicine, and the USC Tina and Rick Caruso Department of Otolaryngology – Head and Neck Surgery, and one of the corresponding authors of the study. “Aging, loud noises, and certain chemotherapy drugs and antibiotics can all lead to the permanent loss of hair cells, which is the leading contributor to hearing loss worldwide.”
iHCs have the potential to accelerate hearing loss research in at least two important ways, according to Ichida, who is the John Douglas French Alzheimer’s Foundation Associate Professor of Stem Cell Biology and Regenerative Medicine at USC, and the other corresponding author of the study.
“In the near term, researchers can use iHCs to screen large numbers of drug candidates that might prevent or treat hearing loss,” said Ichida, who is also a New York Stem Cell Foundation-Robertson Investigator. “And further in the future, it could become possible to directly reprogram supporting cells in the inner ear of a deafened individual, as a way to restore hearing.”
###
This paper is the result of a collaboration between the Segil and Ichida labs, initiated by Menendez and Suhasni Gopalakrishnan. Two additional groups joined the collaboration to characterize the new iHCs: Radha Kalluri, assistant professor in the USC Tina and Rick Caruso Department of Otolaryngology – Head and Neck Surgery, and Alex Markowitz characterized the physiological properties of the new cells; and James Lee and Chichou Huang of DRVision Technologies developed software for robotically imaging and quantifying the growth and death of the iHCs. Other key contributors from the Segil Lab were Talon Trecek and Litao Tao, who collected and analyzed data, as well as Juan Llamas, Xizi Wang, and Haoze Vincent Yu.
The collaboration was catalyzed by a Regenerative Medicine Initiative grant from the Office of the Dean of the Keck School of Medicine of USC. Seventy percent of this work was supported by federal funding from the National Institutes of Health (R01DC015530, R00NS077435, and R01NS097850). Additional sources of funding include the New York Stem Cell Foundation and the Merkin Family Foundation.
Media Contact
Cristy Lytal
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




