TMIMS and iCONM started joint research
Credit: 2020 Innovation Center of NanoMedicine
June 4, 2020 – Kawasaki / Japan: The Innovation Center of NanoMedicine (President: Prof. Kazunori Kataoka, location: Kawasaki in Japan; abbreviation: iCONM) initiated a collaborative study with the Tokyo Metropolitan Institute of Medical Science (President: Dr. Hisao Masai, location: Tokyo in Japan, abbreviation: TMIMS) on April 1st, 2020, aiming to rapidly develop mRNA vaccine incorporated with immunostimulatory adjuvant functionality (Note 1) in preparation for the re-pandemic of covid-19 and the attacks by next coming new coronaviruses. In iCONM, the Kawasaki base of “Center of Innovation Program” granted by the Japan Science and Technology Agency (JST) of the Ministry of Education, Culture, Sports, Science and Technology, “In-Body Hospitals” project has been proceeding since 2013 (See: https:/
Seven coronaviruses are currently known to infect humans, four of which are common cold viruses and not very serious ones. However, the outbreak of other three coronaviruses; SARS (severe acute respiratory syndrome) in 2002, MERS (Middle East respiratory syndrome) in 2012, and covid-19 this year, demonstrated coronavirus subspecies occurring approximately every decade, suggesting that new coronavirus variants are likely to occur in near future.
The aim of this collaborative study is to adapt iCONM’s basic technology, mRNA loaded smart nanomachines (Note 3), to the TMIMS’s expertise for the discovery of recombinant vaccines, and to develop a technology that can efficiently produce a vaccine in a short period of time and at a low cost in preparation for the return of covid-19 and the attack of further new coronavirus that will likely arise in the future.
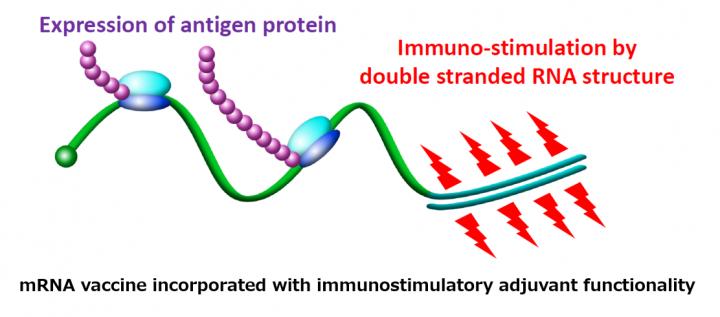
(Note 1) mRNA vaccine incorporated with immunostimulatory adjuvant functionality: In order for mRNA vaccine to function, it is required not only to efficiently introduce mRNA into antigen-presenting cells but also to activate them for effective antibody production. Substances that enhance the action of vaccines in this way are called “adjuvants.”
The introduction of adjuvants and mRNA into the same antigen-presenting cells enables them to effectively induce immunity to the antigen.
S. Uchida, N. Yoshinaga, K. Yanagihara, E. Yuba, K. Kataoka, K. Itaka, “Designing Immunoactivating Double-strand Messenger RNAs Maintaining Translation Activity through Hybridization with Poly A Sequence for Vaccination Effective” Biomaterials 150 162-170 (2018) (DOI: 10.1016/j. biomaterials. 2017.09.033)
(Note 2) Vaccine development using vaccinia virus: The vaccine that contributed to the elimination of smallpox is a live vaccine containing vaccinia virus classified as the same orthopoxvirus as smallpox virus. It is often used as a basis for recombinant vaccines in recent years due to the following reasons: the ability to be administered systemically by intravenous administration, the lack of risk of chromosomal integration without translocation into the cell nucleus, the ability to infect a variety of cancer cells, and the ability to transfer a wide variety of genes with a viral genome size of 200kbp. In TMIMS, recombinant vaccines against H5N1 highly pathogenic avian influenza have been successfully generated using vaccinia virus.
M. Kohara, F. Yasui, H. Kida, Y. Sakoda and K. Ishii, “Recombinant Vaccinia Virus Derived from DIs Strains Harboring Hemagglutinin Protein Genes from New Influenza Virus”. JP5884100B2 (2011)
(Note 3) mRNA-loaded smart nanomachines: Spherical or rod-shaped molecular assembly (micelles) with a size of tens of nm formed by associating amphipathic polymers with various functional molecules in water. 1 nm is one billionth of a meter. If we compare the height of a person to the diameter of the earth, the size of one cell is that of Tokyo Dome, and the size of the smart nanomachine is that of a soccer ball. Unlike DNA, mRNA serves as a blueprint for protein synthesis outside the cell nucleus. Since it is a very unstable compound and has a problem of causing an inflammatory reaction, it is difficult to handle as a drug as it is, but it has been known that these problems can be solved by mounting it on a smart nanomachine.
H. Cabral, K. Miyata, K. Osada, K. Kataoka, Block copolymer micelles in nanomedicine applications. Chem. Rev. 118 (14) 6844-6892 (2018) (DOI: 10.1021/acs.chemrev.8b00199)
S. Uchida, K. Kataoka, Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. A 107 Issue 5 978-990 (2019) (DOI: 10.1002/jbm.a.36614)
###
Innovation Center of NanoMedicine (iCONM):
The Innovation Center of NanoMedicine (iCONM) is a leading facility of King Skyfront, that is a biotech and healthtech innovation cluster in Kawasaki City. iCONM started the operation in April 2015 with Kawasaki Institute of Industrial Promotion in order to drive “Center of Open Innovation Network for Smart Health (COINS)” as a part of Japanese governmental research program “Center of Innovation (COI) Stream”. Designed for the purpose of promoting “open innovation” through industry-academia-government and medicine-engineering collaborations with state-of-the-art facilities and experimental equipment capable of conducting the R&D from organic synthesis and micro-processing to preclinical studies. This is a very unique research center that is hardly found in the world.
https:/
Tokyo Metropolitan Institute of Medical Science (TMIMS):
In April 2011, three separate Tokyo research institutes were combined to form the Tokyo Metropolitan Institute of Medicine, and the Governor of Tokyo certified our institute as a public benefit foundation on April 1, 2012. By conducting basic and medical research comprehensively, and translating these results into therapies, we aim to improve the medical care and contribute to the well-being of the metropolitan population.
http://www.
Media Contact
Makoto Shimazaki, Ph. D.
[email protected]




