With Epidax, COVID-19 testing can be done at the point of care, and diagnosis — from sample collection to result – can be completed in about an hour
Credit: National University of Singapore
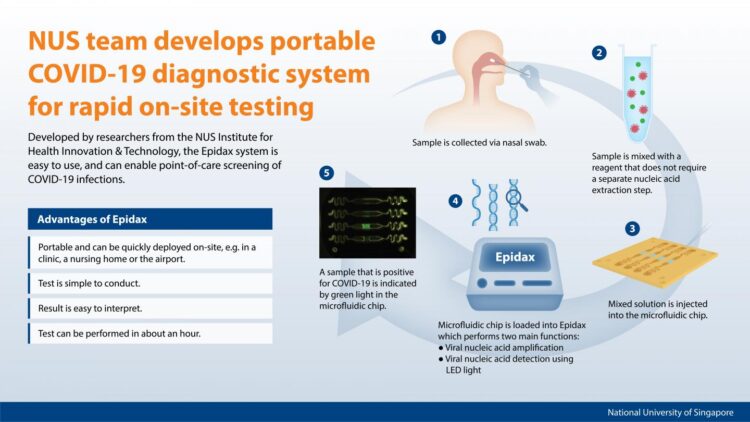
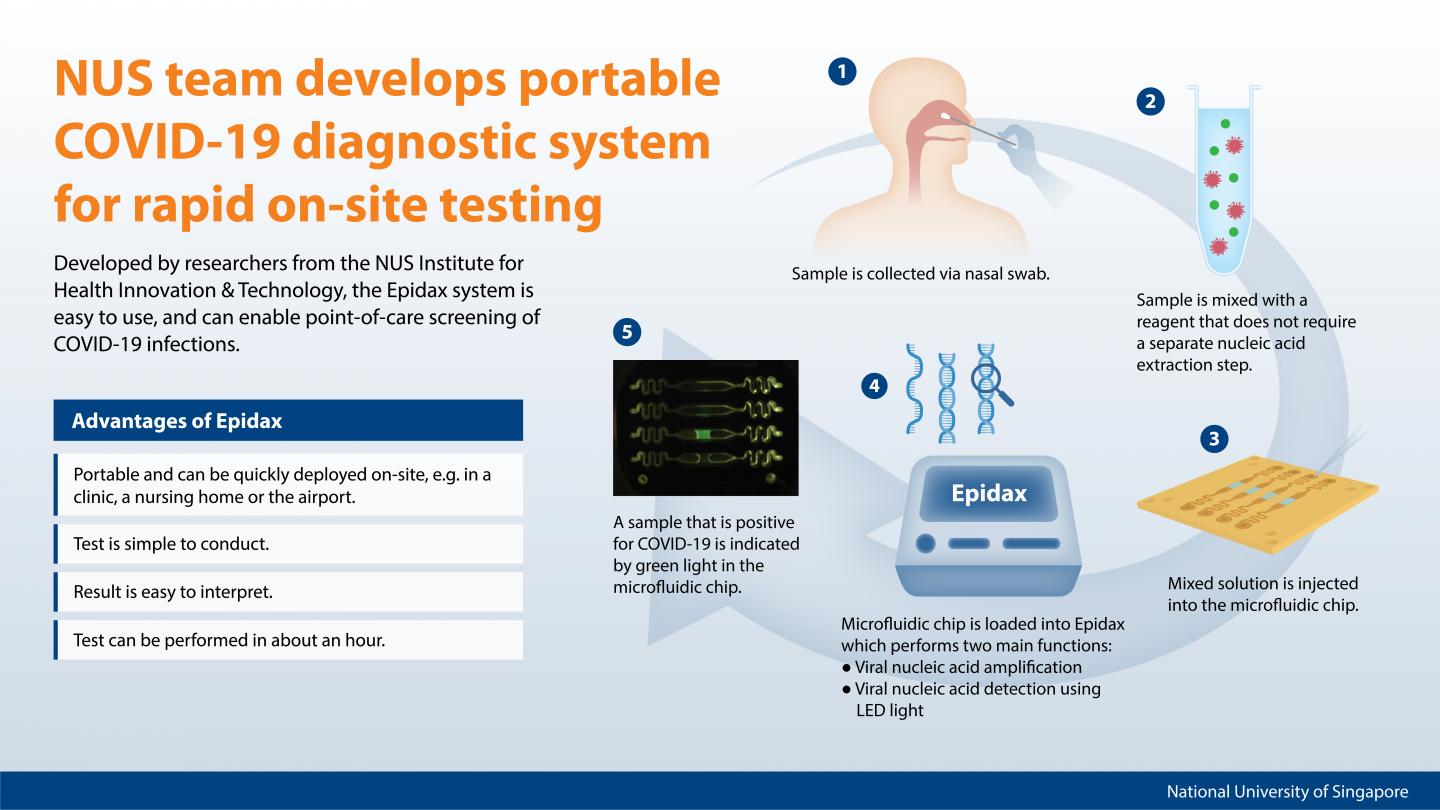
COVID-19 screening can soon be conducted directly at various testing stations, and patients can get their test results in about an hour from the time they get a nasal swab. A team of researchers from the National University of Singapore (NUS) has developed a portable COVID-19 micro-PCR diagnostic system – called Epidax – that enables rapid and accurate on-site screening of infectious diseases and significantly reduces the time required to analyse patient samples.
Polymerase chain reaction (PCR) test that is currently being used for COVID-19 diagnosis has to be carried out in specialised testing facilities, and takes a few hours or a few days for results to be made known.
A 10-member team from the NUS Institute for Health Innovation & Technology (iHealthtech), led by the institute’s Director Professor Lim Chwee Teck, has developed a novel diagnostic system from scratch in a record time of two months. A project of this scale would typically take at least one to two years to complete.
“The COVID-19 pandemic has posed unprecedented health, economic and social challenges around the world. Until a vaccine against the coronavirus becomes available, ramping up testing is a critical first step to curb the spread of the disease and to provide appropriate medical care for those tested positive. However, these tests take time and come at a high cost in terms of manpower and equipment. If we can quickly detect the coronavirus, we can better contain it. Epidax provides an effective solution to address these limitations,” said Prof Lim.
Portable system, easy to operate
Epidax, which is a microfluidics-based PCR diagnostic system, is about the size of a toaster and very portable. It can be deployed quickly and easily on-site for virus infection screening.
Currently, nasal swab samples are first collected at a clinic or testing site, and sent to a laboratory for processing to extract the RNA, before the PCR test is conducted.
The Epidax system uses a specially designed microfluidic chip that comprises micro-channels where samples are processed. By employing microfluidic technology, the system is able to process a smaller amount of sample for quicker detection of COVID-19 infection. Using a reagent which enables both RNA extraction and amplification on the chip, the PCR test can be performed right after a nasal swab sample is collected, thus bypassing the intermediate step of RNA extraction. All these features significantly minimise sample handling and shorten the test and waiting time, so patients can get their test results in about an hour.
“We have designed the Epidax system to be very easy to use. The lab technician operating the system only needs to pipette the sample and reagent into the microfluidic chip and load it into the Epidax system for processing. These simple steps can be easily executed within 5 minutes,” said Prof Lim.
The NUS research team validated the Epidax system against existing PCR systems, and found that the Epidax system has the same or even higher sensitivity than some of the current PCR systems. In fact, the sensitivity of detection can achieve at least 10 copies of RNA per microlitre of sample. The team is currently improving the limit of detection, aiming to reach 1 copy of RNA per microlitre of sample.
Prof Lim explained, “The standard PCR diagnostic test is currently the gold standard test conducted in a centralised laboratory to identify SARS-CoV-2 viral infection. Our Epidax system is a unique microfluidic chip-based diagnostic system that can conduct PCR test on-site for the rapid screening of infection, whether it is at a local clinic, a nursing home or the airport. In this way, we can quickly identify infected individuals and take swift action to prevent transmission.”
The development of Epidax was not without challenges. “The research was carried out during the circuit breaker period. We had to overcome various constraints, such as supply chain disruption, shortage of consumables and components, as well as restrictions on access and movement due to the additional safety measures that had to be put in place. However, the team persevered to overcome all odds to complete the project, demonstrating resourcefulness and amazing teamwork,” said Dr Nguyen Quoc Mai Phuong, who is the co-project leader.
Commercialisation of Epidax
The NUS team has filed a patent for this invention, and is in talks with a medical technology company to commercialise this technology.
Prof Lim said, “Moving forward, we are keen to further develop our portable micro-PCR diagnostic system that can even be deployed at home. For example, it can resemble a small capsule coffee machine: portable, affordable, easy to use, and we can insert different ‘capsules’ to test for a variety of diseases. With the current advances in science and technology, I believe this is highly achievable in the near future.”
The Epidax system is one of the innovations developed by NUS to tackle the current global pandemic. Since the start of the crisis, the University has been proactively participating in the fight against COVID-19 on different fronts, with research ranging from rapid diagnostics to case connections and vaccine development, as well as harnessing information and technology solutions to model public health, fight false rumours online and more.
###
Media Contact
Fun Yip
[email protected]
Original Source
https:/