Rice U. lab unveils catalyst that can break problematic C-F bonds
Credit: H. Robatjazi/Rice University
HOUSTON — (June 22, 2020) — Rice University engineers have created a light-powered catalyst that can break the strong chemical bonds in fluorocarbons, a group of synthetic materials that includes persistent environmental pollutants.
In a study published this month in Nature Catalysis, Rice nanophotonics pioneer Naomi Halas and collaborators at the University of California, Santa Barbara (UCSB) and Princeton University showed that tiny spheres of aluminum dotted with specks of palladium could break carbon-fluorine (C-F) bonds via a catalytic process known as hydrodefluorination in which a fluorine atom is replaced by an atom of hydrogen.
The strength and stability of C-F bonds are behind some of the 20th century’s most recognizable chemical brands, including Teflon, Freon and Scotchgard. But the strength of those bonds can be problematic when fluorocarbons get into the air, soil and water. Chlorofluorocarbons, or CFCs, for example, were banned by international treaty in the 1980s after they were found to be destroying Earth’s protective ozone layer, and other fluorocarbons were on the list of “forever chemicals” targeted by a 2001 treaty.
“The hardest part about remediating any of the fluorine-containing compounds is breaking the C-F bond; it requires a lot of energy,” said Halas, an engineer and chemist whose Laboratory for Nanophotonics (LANP) specializes in creating and studying nanoparticles that interact with light.
Over the past five years, Halas and colleagues have pioneered methods for making “antenna-reactor” catalysts that spur or speed up chemical reactions. While catalysts are widely used in industry, they are typically used in energy-intensive processes that require high temperature, high pressure or both. For example, a mesh of catalytic material is inserted into a high-pressure vessel at a chemical plant, and natural gas or another fossil fuel is burned to heat the gas or liquid that’s flowed through the mesh. LANP’s antenna-reactors dramatically improve energy efficiency by capturing light energy and inserting it directly at the point of the catalytic reaction.
In the Nature Catalysis study, the energy-capturing antenna is an aluminum particle smaller than a living cell, and the reactors are islands of palladium scattered across the aluminum surface. The energy-saving feature of antenna-reactor catalysts is perhaps best illustrated by another of Halas’ previous successes: solar steam. In 2012, her team showed its energy-harvesting particles could instantly vaporize water molecules near their surface, meaning Halas and colleagues could make steam without boiling water. To drive home the point, they showed they could make steam from ice-cold water.
The antenna-reactor catalyst design allows Halas’ team to mix and match metals that are best suited for capturing light and catalyzing reactions in a particular context. The work is part of the green chemistry movement toward cleaner, more efficient chemical processes, and LANP has previously demonstrated catalysts for producing ethylene and syngas and for splitting ammonia to produce hydrogen fuel.
Study lead author Hossein Robatjazi, a Beckman Postdoctoral Fellow at UCSB who earned his Ph.D. from Rice in 2019, conducted the bulk of the research during his graduate studies in Halas’ lab. He said the project also shows the importance of interdisciplinary collaboration.
“I finished the experiments last year, but our experimental results had some interesting features, changes to the reaction kinetics under illumination, that raised an important but interesting question: What role does light play to promote the C-F breaking chemistry?” he said.
The answers came after Robatjazi arrived for his postdoctoral experience at UCSB. He was tasked with developing a microkinetics model, and a combination of insights from the model and from theoretical calculations performed by collaborators at Princeton helped explain the puzzling results.
“With this model, we used the perspective from surface science in traditional catalysis to uniquely link the experimental results to changes to the reaction pathway and reactivity under the light,” he said.
The demonstration experiments on fluoromethane could be just the beginning for the C-F breaking catalyst.
“This general reaction may be useful for remediating many other types of fluorinated molecules,” Halas said.
###
Halas is the Stanley C. Moore Professor of Electrical and Computer Engineering in Rice’s Brown School of Engineering, director of Rice’s Smalley-Curl Institute and a professor of chemistry, bioengineering, physics and astronomy, and materials science and nanoengineering.
Additional co-authors include Ming Zhang, Linan Zhou and Peter Nordlander, all of Rice; Junwei Lucas Bao, formerly of Princeton University and now at Boston College; Emily Carter, formerly of Princeton and now at UCLA; and Phillip Christopher of UCSB.
This research was supported by the Air Force Office of Scientific Research (MURI FA9550-15-1-0022), the Defense Threat Reduction Agency (HDTRA1-16-1-0042), the Welch Foundation (C-1220 and C-1222) and the Arnold and Mabel Beckman Foundation.
Links and resources:
The DOI of the Nature Catalysis paper is: 10.1038/s41929-020-0466-5
A copy of the paper is available at: https:/
High-resolution IMAGES are available for download at:
https:/
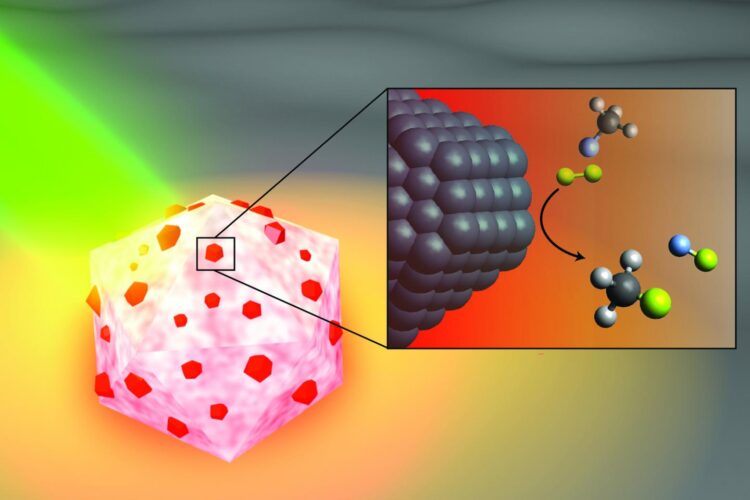
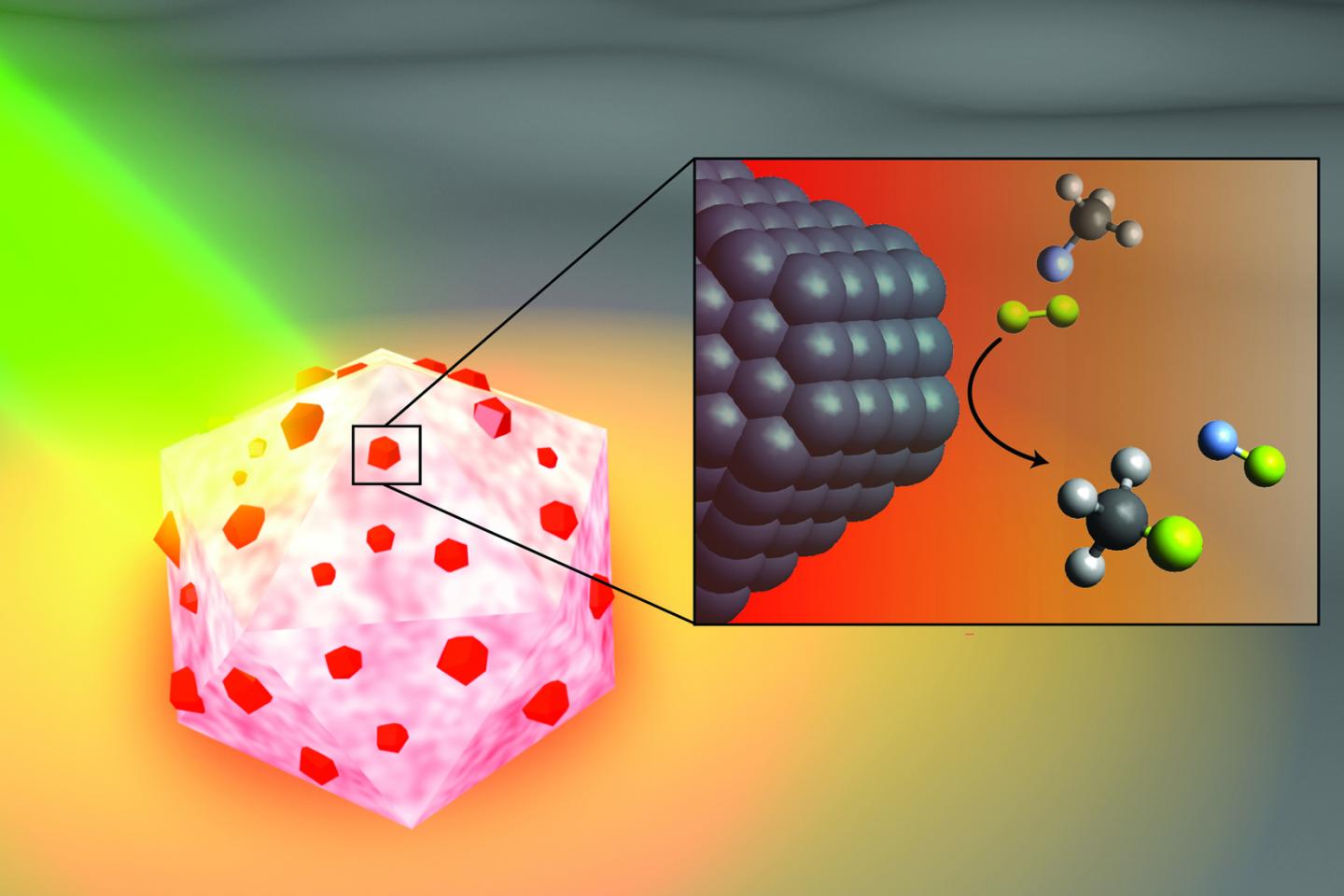
CAPTION: An artist’s illustration of the light-activated antenna-reactor catalyst Rice University engineers designed to break carbon-fluorine bonds in fluorocarbons. The aluminum portion of the particle (white and pink) captures energy from light (green), activating islands of palladium catalysts (red). In the inset, fluoromethane molecules (top) comprised of one carbon atom (black), three hydrogen atoms (grey) and one fluorine atom (light blue) react with deuterium (yellow) molecules near the palladium surface (black), cleaving the carbon-fluorine bond to produce deuterium fluoride (right) and monodeuterated methane (bottom). (Image courtesy of H. Robatjazi/Rice University)
https:/
CAPTION: Hossein Robatjazi is a Beckman Postdoctoral Fellow at the University of California, Santa Barbara. (Photo courtesy of H. Robatjazi/UCSB)
https:/
CAPTION: Rice University’s Naomi Halas is an engineer, chemist and pioneer in the field of light-activated nanomaterials. (Photo by Jeff Fitlow/Rice University)
This release can be found online at news.rice.edu.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Media Contact
Jade Boyd
[email protected]
Related Journal Article
http://dx.