Researchers develop a new way to create 3D scaffolds for biomedical applications
Credit: Jingwei Xie
WASHINGTON, May 12, 2020 — In the movie “Transformers,” cars morph into robots, jets or a variety of machinery. A similar concept inspired a group of researchers to combine gas foaming, which is a blend of chemicals that induces gas bubbling, and 3D molding technologies to quickly transform electrospun membranes into complex 3D shapes for biomedical applications.
In Applied Physics Reviews, from AIP Publishing, the group reports on its new approach that demonstrates significant improvements in speed and quality compared with other methods. The work is also the first successful demonstration of formation of 3D neural tissue constructs with an ordered structure through differentiation of human neural progenitor/stem cells on these transformed 3D nanofiber scaffolds.
“Electrospinning is a technology to produce nanofiber membranes,” said co-author Jingwei Xie, at the University of Nebraska Medical Center. “The physics principle behind it involves applying an electrical force to overcome the surface tension of a solution to elongate a solution jet into continuous and ultrafine fibers after solvent evaporation.”
Due to an intrinsic property of electrospinning, nanofibers are often deposited to form 2D membranes or sheets with dense structures and small pore sizes that are less than the size of cells.
“This greatly inhibits the applications of electrospun nanofibers, because cells fail to seed or penetrate throughout the nanofiber membranes, which is undesirable,” he explained.
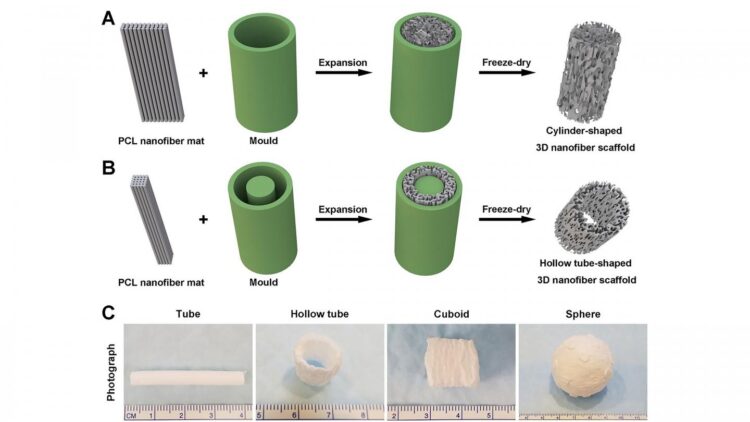
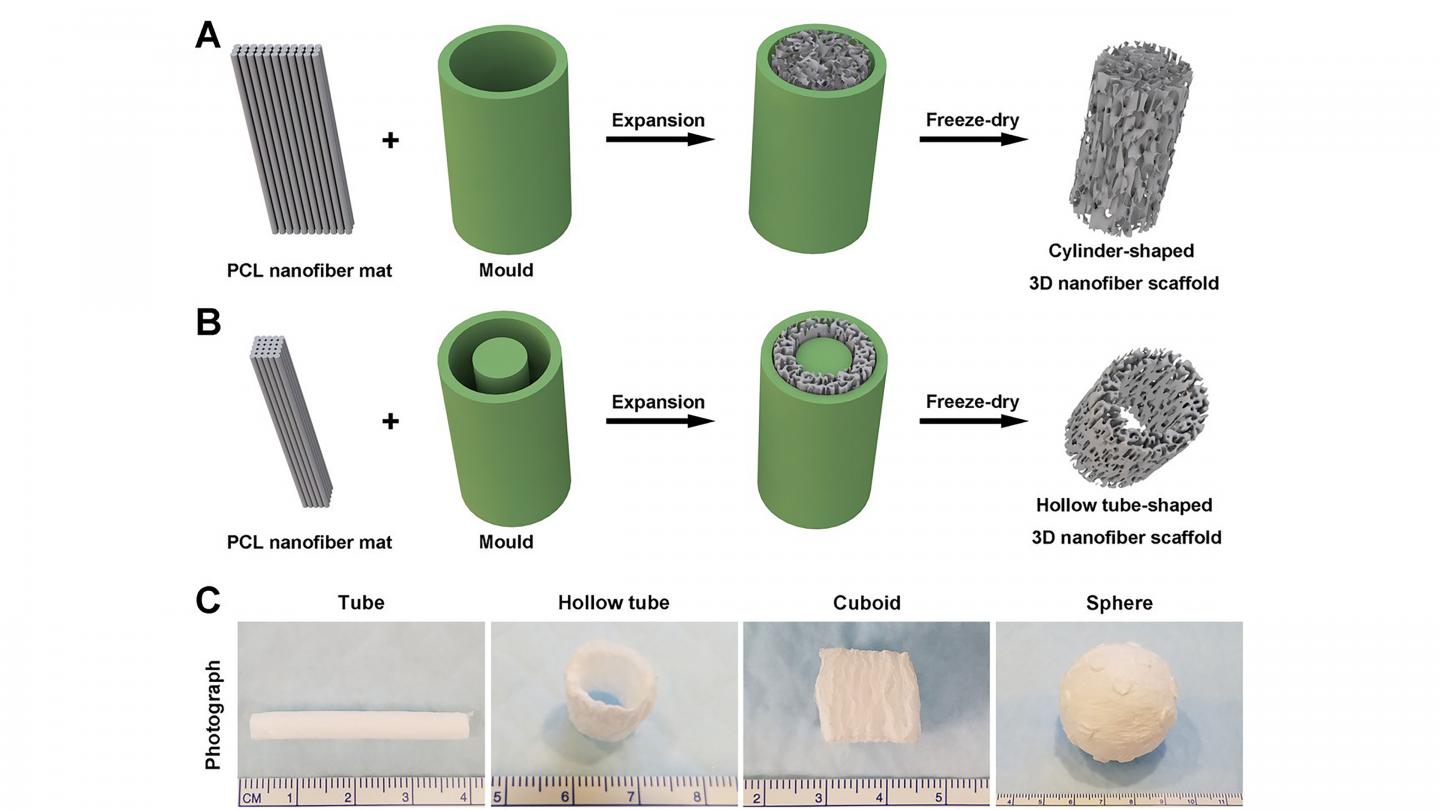
Researchers combined gas foaming and 3D molding concepts to expand nanofiber membranes within a confined space to form predesigned 3D nanofiber objects in cylindrical, cuboid, spherical, and irregular shapes.
“Our 3D objects have the appropriate pore size and controlled fiber alignment for guiding and enhancing cell penetration to form new tissue,” Xie said.
The group’s work is significant, because it can be done within an hour. Other methods can require up to 12 hours to complete the transformation process.
“Thanks to the ability to mimic the architecture of extracellular matrix, electrospun nanofibers show great potential in applications such as tissue engineering, regenerative medicine and tissue modeling,” said Xie.
One of the group’s most intriguing findings is that after coating 3D nanofiber objects with gelatin, they exhibit superelasticity and shape recovery.
“Gelatin-coated, cube-shaped scaffolds functionalized with polypyrrole coatings exhibited dynamic electrical conductivity during cyclical compression,” he said.
They also demonstrated that cuboid-shaped nanofiber objects were effective for compressible hemorrhage in a pig liver injury model.
In the future, the group’s method may help “enable therapeutic-free biomaterials for tissue repair and regeneration, such as using predesigned nanofiber objects to fit irregular tissue defects,” Xie said. “Beyond that, superelasticity and shape recovery could allow 3D-nanofiber objects to be applied in a minimally invasive manner.”
###
The article, “Fast transformation of 2D nanofiber membranes into pre-molded 3D scaffolds with biomimetic and oriented porous structure for biomedical applications,” is authored by Sixuan Chen, Johnson V. John, Alec McCarthy, Mark A. Carlson, Xiaowei Li and Jingwei Xie. It will appear in Applied Physics Reviews, May 12, 2020 (DOI: 10.1063/1.5144808). After that date, it can be accessed at https:/
ABOUT THE JOURNAL
Applied Physics Reviews features articles on significant and current topics in experimental or theoretical research in applied physics, or in applications of physics to other branches of science and engineering. The journal publishes both original research on pioneering studies of broad interest to the applied physics community, and reviews on established or emerging areas of applied physics. See https:/
Media Contact
Larry Frum
[email protected]
Related Journal Article
http://dx.