Credit: Hyung Joon Cha (POSTECH)
Early mortality of myocardial infarction (MI), one of fatal diseases, is about 30%. So, it is critical to have immediate and proactive treatment to prevent a heart attack. Contributing to developing an efficient treatment of this fatal disease, a research team from South Korea recently proposed an effective stem cell treatment system for myocardial infarction, using harmless protein from mussel and stem cells.
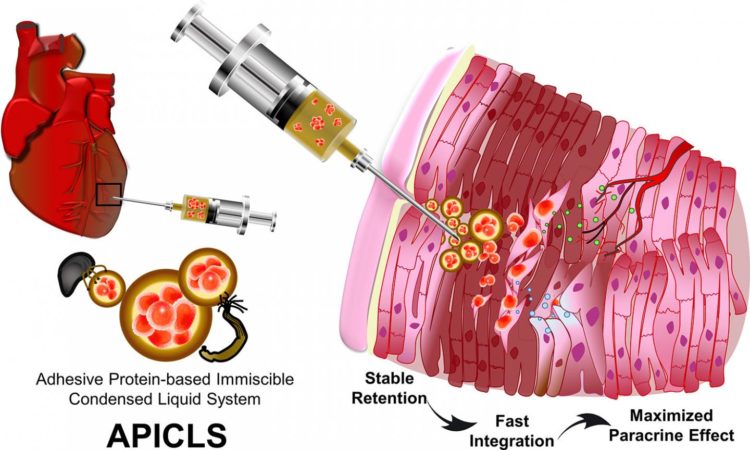
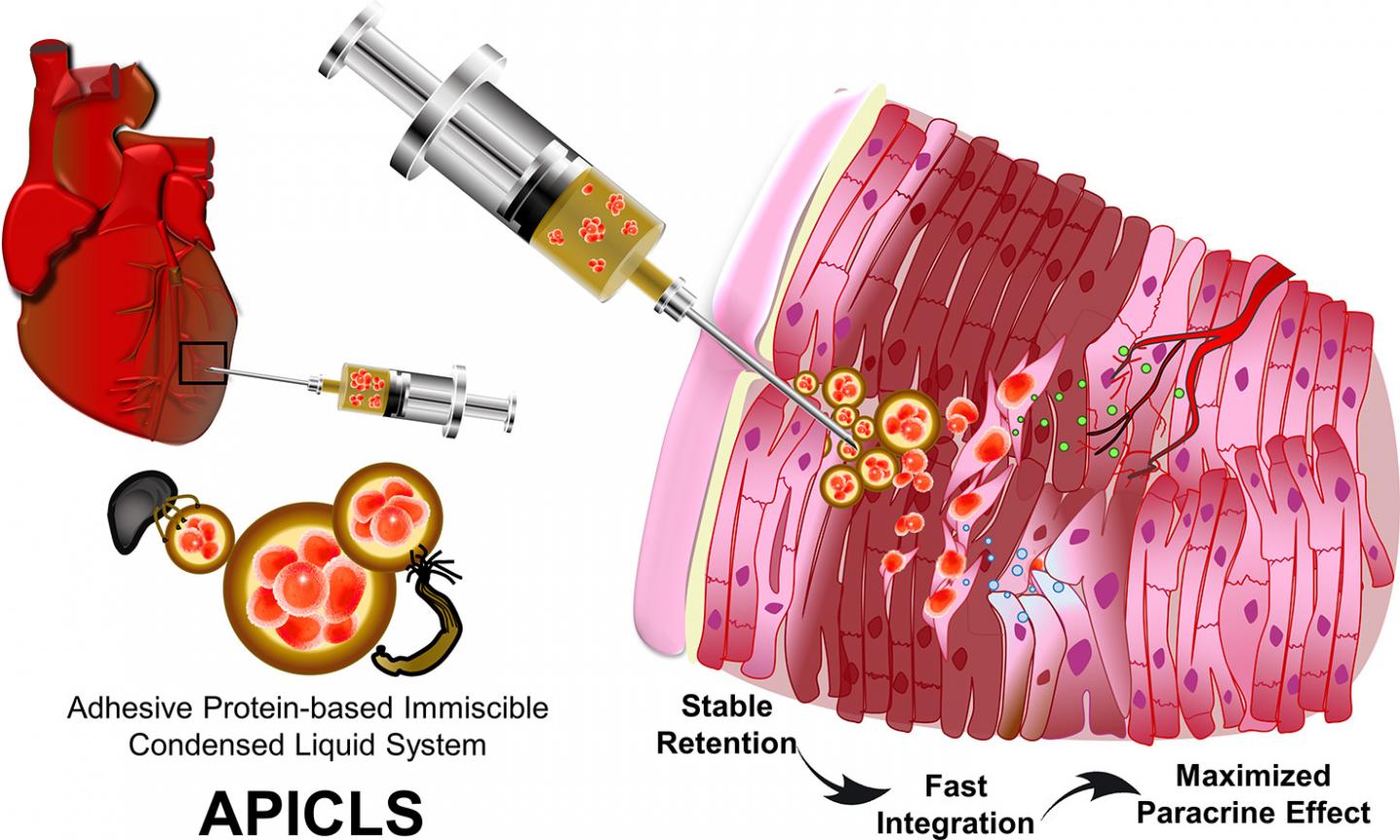
Prof. Hyung Joon Cha and Mr. Tae Yoon Park from Department of Chemical Engineering, POSTECH with Prof. Sung Bo Sim from Department of Thoracic and Cardiovascular Surgery, Yeouido St. Mary’s Hospital and Prof. Jongho Lee from Department of Thoracic and Cardiovascular Surgery, Daejeon St. Mary’s Hospital developed an ‘adhesive protein-based immiscible condensed liquid system’ (APICLS) that efficiently delivered the mesenchymal stem cells (MSCs) to the damaged cardiac muscular tissues and enabled the transplantation prolonged. By employing the phase separation phenomenon of mussel adhesive protein, they were able to easily encapsulate the MSCs in the liquid coacervate. Especially, based on the mass production of bioengineered mussel adhesive protein, their newly suggested platform can be expected to be an innovative therapeutic system for myocardial infarction.
Heart is a vital organ that circulates blood while repeating contraction and relaxation of muscles by electrical signals. When blood vessels are clogged, oxygens and nutrients cannot be supplied to the heart and it brings severe damages to a muscle of the heart, causing infarcted myocardium with disruption of blood networks. This causes a necrosis on wall of the myocardium, resulting in cardiac wall thinning and this phenomenon is known as myocardial infarction. Because the heart cannot regenerate itself when it is damaged, there is no method for innovatively regenerating damaged heart muscles. As current therapeutic strategies, patients are treated with either mechanical device or heart transplantation.
Recently, there have been numbers of research proposing on transplanting exogenous stem cells into the damaged myocardium to help heart regeneration as a future treatment technique. However, transplanted stem cells have very low survival rate due to harsh environment of the heart. Even when the transplantation is successful, most of the stems cells soon die.
For a successful stem cell therapy on MI, there are two conditions required to survive in harsh environment of the damaged heart. First, the stem cells must be efficiently transplanted and remained into the thinned cardiac muscles. Secondly, transplanted stem cells must integrate rapidly into resident surrounding tissues to improve their viability by forming blood vessels. However, the current therapeutic methods so far cannot deliver injected stem cells to infarcted cardiac muscular tissues successfully, making it very difficult to maintain the transplantation.
The joint research team injected the MSCs encapsulated in APICLS into the thinned and infarcted cardiac muscular wall efficiently. They demonstrated in vivo feasibility through rat MI model that transplanted MSCs survived in the infarcted cardiac muscular tissues for a long time due to the mussel adhesive proteins with its unique characteristics of adhesiveness and angiogenesis and the efficacy of MSCs. Furthermore, the damaged heart muscles formed new blood vessels, prevented further apoptosis of existing cardiomyocytes, and regenerated the damaged cardiac wall by reducing fibrosis.
It is anticipated that the new stem cell delivery system proposed in this research will play an essential role in the stem cell therapeutic market as it used biocompatible materials which are harmless to humans.
“By using mussel adhesive proteins, we demonstrated with the MI rat model and proved its therapeutic efficacy as an efficient stem cell injection strategy. We gives a hope that it can also be successfully applied to chronic diseases and ischemic diseases that have similar environment,” said Prof. Hyung Joon Cha who led the research.
In the meanwhile, this research was introduced as the most innovative technology found by POSTECH in the Most Innovative Universities 2019 by Reuters last year. It is also published on the website of Journal of Controlled Release, the world’s most renowned journal in the field of drug delivery. This study was supported by the Marine BioMaterials Research Center grant funded by the Ministry of Oceans and Fisheries, Korea.
###
Media Contact
Jinyoung Huh
[email protected]
82-542-792-415
Original Source
http://postech.
Related Journal Article
http://dx.