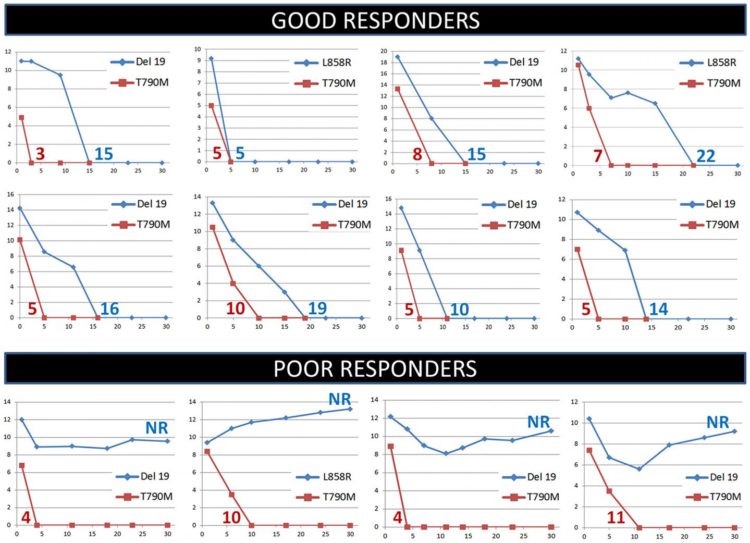
Oncotarget Volume 11, Issue 11 reported that at clinical progression, 64 EGFR T790M plasma positive patients were subjected to second line-treatment with osimertinib and strictly monitored during the first month of therapy.
Credit: Correspondence to – Antonio Marchetti – [email protected]
Oncotarget Volume 11, Issue 11 reported that at clinical progression, 64 EGFR T790M plasma positive patients were subjected to second line-treatment with osimertinib and strictly monitored during the first month of therapy.
Plasma analysis by the EGFR Cobas test showed in 57 cases a substantial decrease in the levels of the sensitizing EGFR mutant allele, down to a not detectable value.
The research team’s data indicate that plasma monitoring by a simple RT-PCR-based EGFR mutation test in the first month of treatment may be useful for a rapid identification of patients to be subjected to further characterization by MPS.
Dr. Antonio Marchetti from the Laboratory of Diagnostic Molecular Oncology, Center for Advanced Studies and Technology (CAST) and The Department of Medical and Oral Sciences and Biotechnologies at the University of Chieti as well as The Department of Pathology, SS Annunziata Clinical Hospital said, “In Non-Small-Cell Lung Cancer (NSCLC) patients treated with Tyrosine Kinase-Inhibitors (TKIs) therapy, the emergence of acquired resistance can be investigated by plasma monitoring of circulating tumor DNA (ctDNA)“
“In Non-Small-Cell Lung Cancer (NSCLC) patients treated with Tyrosine Kinase-Inhibitors (TKIs) therapy, the emergence of acquired resistance can be investigated by plasma monitoring of circulating tumor DNA (ctDNA)”
– Dr. Antonio Marchetti, Laboratory of Diagnostic Molecular Oncology, Center for Advanced Studies and Technology (CAST), The Department of Medical and Oral Sciences and Biotechnologies at the University of Chieti, & The Department of Pathology, SS Annunziata Clinical Hospital
Several studies have shown a high concordance between the presence of EGFR mutations in tissue and plasma, especially in patients with diffuse metastatic disease.
In a previous study, the authors have shown for the first time that NSCLC patients carrying EGFR mutations in tumor tissue and subjected to first-line treatment with EGFR TKIs, can be strictly monitored in the first days of treatment by repeated blood draws to quantify EGFR mutant alleles in plasma.
These results strongly suggest that the amount of EGFR sensitizing mutations in plasma reflects the tumor burden and that the fluctuations in the levels of EGFR sensitizing mutations in plasma are closely related to tumor load variations.
However, after a medium period of about 12 months, even patients subjected to second-line treatment with osimertinib, develop resistance with various mechanisms, including EGFR SNV, MET, and HER2 amplification, genetic fusions, etc. Recently, a series of clinical trials led to the approval of first-line treatment of EGFR-positive NSCLC patients with osimertinib.
The Marchetti Research Team concluded in their Oncotarget Research paper, “our results indicate that a subset of NSCLC patients subjected to second-line treatment with osimertinib are resistant to treatment due to the presence of different types of mutations. Plasma monitoring by a simple RT-PCR-based EGFR mutation test in the first month of treatment may be useful to rapidly identify these cases and subject them to MPS analysis for further characterization and treatment.“
Sign up for free Altmetric alerts about this article
DOI – https:/
Full text – http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path[]=27517&path[]=90092
Correspondence to – Antonio Marchetti – [email protected]
Keywords –
epidermal growth factor receptor (EGFR),
tyrosine-kinase Inhibitors,
circulating tumor-DNA (ct-DNA),
massive parallel sequencing (MPS),
resistance-inducing mutation
About Oncotarget
Oncotarget is a weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit http://www.
SoundCloud – https:/
Facebook – https:/
Twitter – https:/
LinkedIn – https:/
Pinterest – https:/
Reddit – https:/
Oncotarget is published by Impact Journals, LLC please visit http://www.
Media Contact
[email protected]
18009220957×105
Media Contact
@RYANJAMESJESSUP
[email protected]
202-638-9720
Original Source
http://www.
Related Journal Article
http://dx.