The cover for issue 11 of Oncotarget features Figure 6, “Effects of AZD8186 in combination with anti-PD1 on syngeneic models,” by Owusu-Brackett, et al.
Credit: Correspondence to – Funda Meric-Bernstam – [email protected]
The cover for issue 11 of Oncotarget features Figure 6, “Effects of AZD8186 in combination with anti-PD1 on syngeneic models,” by Owusu-Brackett, et al.
In vitro cell viability assay and immunoblotting demonstrated that PTEN loss was significantly correlated with AZD8186 sensitivity in triple-negative breast cancer cell lines.
AZD8186 in combination with paclitaxel, eribulin had synergistic effects on growth inhibition in PTEN loss cells.
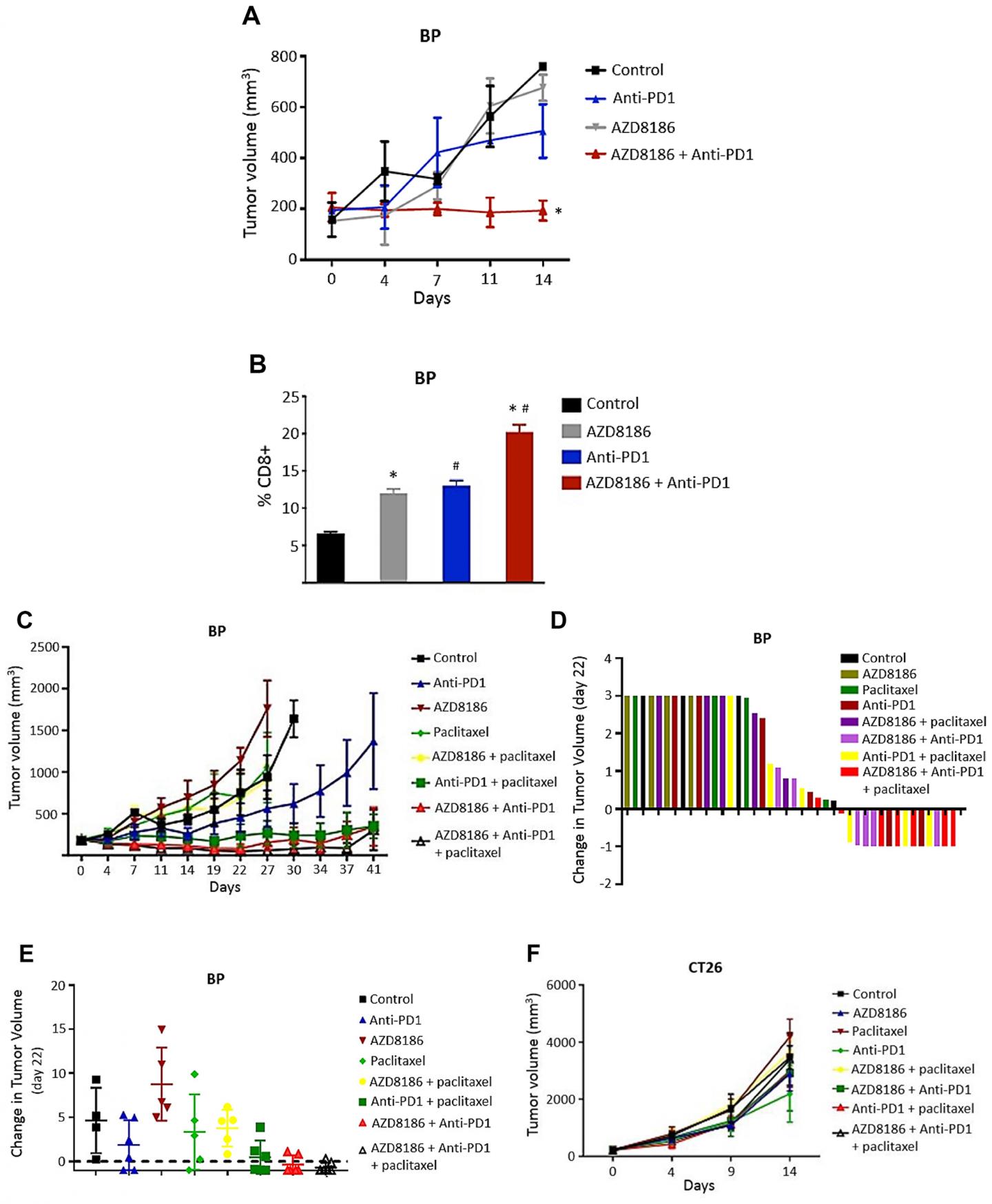
AZD8186 significantly enhanced the antitumor efficacy of anti-PD1 antibodies in the PTEN-deficient BP murine melanoma xenograft model, but not in the PTEN-wild-type CT26 xenograft model.
In vitro, cell proliferation and colony formation assays were performed to determine cell sensitivity to AZD8186.
AZD8186 has single-agent efficacy in PTEN-deficient TNBC cell lines in vitro but has limited single-agent efficacy in vivo.
Dr. Funda Meric-Bernstam from the Department of Surgical Oncology, the Department of Investigational Cancer Therapeutics, the Department of Breast Surgical Oncology, as well as The Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy at The University of Texas MD Anderson Cancer Center, in Houston, Texas, USA said, “Phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway is an important regulator of many physiological cellular processes that promote differentiation, proliferation and survival of a normal cell.“
“Phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway is an important regulator of many physiological cellular processes that promote differentiation, proliferation and survival of a normal cell.”
– Dr. Funda Meric-Bernstam, Department of Investigational Cancer Therapeutics, the Department of Breast Surgical Oncology & The Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy
Mutations, loss of copy number, epigenetic silencing and downregulation of PTEN protein by mi RNA can result in PTEN function inactivation, leading to activation of PI3K/AKT/mTOR pathway, which subsequently increases tumor growth, invasion and metastasis across a diverse set of solid tumors including breast, endometrial, prostate, renal cell, hepatocellular, glioblastoma, and colorectal cancers.
Loss of PTEN and increased PI3K signaling are associated with resistance to trastuzumab and endocrine therapy in hormone receptor-positive breast cancer and with poor prognosis in triple-negative breast cancers.
In vitro, they revealed significant growth inhibition of PTEN-deficient tumors by depleting PIK3CB which encodes PI3K, while no such growth inhibition effect was shown in corresponding PTEN-deficient tumors with downregulation of PIK3CA or PIK3CD encoding PI3K and PI3K, respectively.
Thus, PI3K isoform is the driver of abnormal proliferation in PTEN-null cancers, and as such, PI3K is a promising target for therapy in PTEN-deficient TNBC. AZD8186 is a selective and potent small-molecule inhibitor of PI3K, with additional activity against PI3K isoform.
The Meric-Bernstam Research Team concluded in their Oncotarget Research Paper, “these results provide preclinical evidence of antitumor efficacy of AZD8186 in PTEN-deficient solid tumors. AZD8186 has single-agent efficacy in PTEN-deficient TNBC cell lines in vitro, with modest single-agent efficacy in vivo. Furthermore, AZD8186 enhanced the antitumor efficacy of paclitaxel but stable and progressive disease were noted with this combination in immunosuppressed models. In immunocompetent models, AZD8186 in combination with anti-PD1 resulted in tumor regression in PTEN-deficient BP tumor. We realize that while there appears to be an association of AZD8186 sensitivity to PTEN loss, a cause-effect relationship can only be speculated on. In summary, although further insights are needed into the mechanisms of activity of these combinations, the combination of AZD8186 with taxanes and with anti-PD1 agents hold promise for the treatment of PTEN-deficient solid tumors.“
Sign up for free Altmetric alerts about this article
DOI – https:/
Full text – http://www.
Correspondence to – Funda Meric-Bernstam – [email protected]
Media Contact
@RYANJAMESJESSUP
[email protected]
202-638-9720
Original Source
http://www.
Related Journal Article
http://dx.